Ginsenoside Rg1
From Metabolomics.JP
(Difference between revisions)
(New page: {{Hierarchy|{{PAGENAME}}}} {{Metabolite |SysName=(3.beta.,6.alpha.,12.beta.)-3,12-Dihydroxydammar-24-ene-6,20-diyl bis-.beta.-D-glucopyranoside |Common Name=&&Ginsenoside Rg1&&Panaxoside ...) |
|||
| (One intermediate revision by one user not shown) | |||
| Line 2: | Line 2: | ||
{{Metabolite | {{Metabolite | ||
| − | |SysName=( | + | |SysName=(3beta,6alpha,12beta)-3,12-Dihydroxydammar-24-ene-6,20-diyl bis-beta-D-glucopyranoside |
| − | |Common Name=&&Ginsenoside Rg1&&Panaxoside A&&Dammarane | + | |Common Name=&&Ginsenoside Rg1&&Panaxoside A&&Dammarane beta-D-glucopyranoside deriv.&&Ginsenoside A2&&Ginsenoside g1&&Panaxoside Rg1&&Sanchinoside C1&&Sanchinoside Rg1&& |
|CAS=22427-39-0 | |CAS=22427-39-0 | ||
|KNApSAcK= | |KNApSAcK= | ||
}} | }} | ||
| + | |||
| + | |||
| + | ==Spectroscopic Data== | ||
| + | |||
| + | {| class="wikitable" style="width:80%" | ||
| + | '''Free alcohol''' | ||
| + | |- | ||
| + | | '''M.P.'''<sup>1</sup> || 194 – 196.5 °C | ||
| + | |- | ||
| + | | '''IR''' (KBr)<sup>1</sup>|| 3380 (OH), 1620 (C=C) cm<sup>-1</sup> | ||
| + | |- | ||
| + | | '''<sup>13</sup>C-NMR''' (C<sub>5</sub>D<sub>5</sub>, 25.15MHz)<sup>2</sup>|| C-1) 39.5, (2) 27.6, (3) 78.6, (4) 40.1, (5) 61.3, (6) 77.8, (7) 44.9, (8) 41.0, (9) 49.9, (10) 39.5, (11) 30.8, (12) 70.3, (13) 48.9, (14) 51.3, (15) 30.6, (16) 26.4, (17) 51.6, (18) 17.4, (19) 17.4, (20) 83.3, (21) 22.3, (22) 35.9, (23) 23.2, (24) 125.8, (25) 130.9, (26) 25.7, (27) 17.7, (28) 31.6, (29) 16.2, (30) 17.0 '''Glc I''' (1) 105.7, (2) 75.3, (3) 80.0, (4) 71.6, (5) 79.3, (6) 62.9 '''Glc II''' (1) 98.1, (2) 74.9, (3) 78.8, (4) 71.3, (5) 77.8, (6) 62.6 | ||
| + | |} | ||
| + | {| class="wikitable" style="width:80%" | ||
| + | '''Deca-O-acetate''' | ||
| + | |- | ||
| + | | '''M.P.'''<sup>1</sup> || 242 - 243 °C | ||
| + | |- | ||
| + | | '''<sup>1</sup>H-NMR''' (CDCl<sub>3</sub>N, 100 MHz)<sup>1</sup>|| 0.96 (s, 3xCH<sub>3</sub>), 1.03 (s, CH<sub>3</sub>), 1.05 (s, CH<sub>3</sub>), 1.18 (s, CH<sub>3</sub>), 1.59 and 1.65 (each s, 3xH-26 and 3xH-27), 1.96 (s, OCOCH<sub>3</sub>), 1.98 (s, 3xOCOCH<sub>3</sub>), 2.01 (s, 3xOCOCH<sub>3</sub>), 2.03 (s, 3xOCOCH<sub>3</sub>), 2.09 (s, OCOCH<sub>3</sub>); (in C<sub>5</sub>D<sub>5</sub>N): 0.97 (s, CH<sub>3</sub>), 1.02 (s, CH<sub>3</sub>), 1.14 (s, CH<sub>3</sub>), 1.25 (s, CH<sub>3</sub>), 1.29 (s, CH<sub>3</sub>), 1.42 (s, CH<sub>3</sub>), 1.68 (3xH-26 and 3xH-27) | ||
| + | |} | ||
| + | <small>1) S. Shibata et al., Tetrahedron, 27, 881 (1971)., 2) O. Tanaka et al., Chem.Pharm.Bull., 27, 88 (1979).</small> | ||
Latest revision as of 11:05, 16 February 2010
Upper classes
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 22427-39-0 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | Ginsenoside Rg1.mol |
| Ginsenoside Rg1 | |
|---|---|
| |
| Structural Information | |
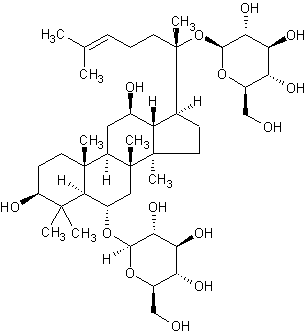
| Systematic Name | (3beta,6alpha,12beta)-3,12-Dihydroxydammar-24-ene-6,20-diyl bis-beta-D-glucopyranoside |
| Common Name |
|
| Symbol | |
| Formula | C42H72O14 |
| Exact Mass | 800.492207012 |
| Average Mass | 801.01268 |
| SMILES | C(C6)(C32C)([H])C(C(C)(C4(C(O)6)[H])CCC(C(OC(C(O)5 |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
[edit] Spectroscopic Data
| M.P.1 | 194 – 196.5 °C |
| IR (KBr)1 | 3380 (OH), 1620 (C=C) cm-1 |
| 13C-NMR (C5D5, 25.15MHz)2 | C-1) 39.5, (2) 27.6, (3) 78.6, (4) 40.1, (5) 61.3, (6) 77.8, (7) 44.9, (8) 41.0, (9) 49.9, (10) 39.5, (11) 30.8, (12) 70.3, (13) 48.9, (14) 51.3, (15) 30.6, (16) 26.4, (17) 51.6, (18) 17.4, (19) 17.4, (20) 83.3, (21) 22.3, (22) 35.9, (23) 23.2, (24) 125.8, (25) 130.9, (26) 25.7, (27) 17.7, (28) 31.6, (29) 16.2, (30) 17.0 Glc I (1) 105.7, (2) 75.3, (3) 80.0, (4) 71.6, (5) 79.3, (6) 62.9 Glc II (1) 98.1, (2) 74.9, (3) 78.8, (4) 71.3, (5) 77.8, (6) 62.6 |
| M.P.1 | 242 - 243 °C |
| 1H-NMR (CDCl3N, 100 MHz)1 | 0.96 (s, 3xCH3), 1.03 (s, CH3), 1.05 (s, CH3), 1.18 (s, CH3), 1.59 and 1.65 (each s, 3xH-26 and 3xH-27), 1.96 (s, OCOCH3), 1.98 (s, 3xOCOCH3), 2.01 (s, 3xOCOCH3), 2.03 (s, 3xOCOCH3), 2.09 (s, OCOCH3); (in C5D5N): 0.97 (s, CH3), 1.02 (s, CH3), 1.14 (s, CH3), 1.25 (s, CH3), 1.29 (s, CH3), 1.42 (s, CH3), 1.68 (3xH-26 and 3xH-27) |
1) S. Shibata et al., Tetrahedron, 27, 881 (1971)., 2) O. Tanaka et al., Chem.Pharm.Bull., 27, 88 (1979).