Index:PK
From Metabolomics.JP
(Difference between revisions)
(New page: {| class="wikitable sortable" ! name || PKS || gene || organism || Ref || Note || |- | 1,3,8-Trihydroxyaceto-Naphthalene | | | Aspergillus p...) |
|||
| Line 1: | Line 1: | ||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
| − | ! name | + | ! name |
| + | !width=10%| PKS | ||
| + | !width=10%| gene | ||
| + | !width=10%| organism | ||
| + | !width=20%| Ref || Note || Size || C2 || | ||
|- | |- | ||
| [[Mol:1,3,8-Trihydroxyaceto-Naphthalene.Mol|1,3,8-Trihydroxyaceto-Naphthalene]] | | [[Mol:1,3,8-Trihydroxyaceto-Naphthalene.Mol|1,3,8-Trihydroxyaceto-Naphthalene]] | ||
| Line 8: | Line 12: | ||
| Herbert1989 | | Herbert1989 | ||
| Compound name have not been confirmed. Naphthalene is the base structure of statins. | | Compound name have not been confirmed. Naphthalene is the base structure of statins. | ||
| − | |||
| | | | ||
| 12 | | 12 | ||
| Line 20: | Line 23: | ||
| Dewick2009 | | Dewick2009 | ||
| acridine alkaloid, starter is Anthranilic acid | | acridine alkaloid, starter is Anthranilic acid | ||
| − | |||
| | | | ||
| | | | ||
| Line 31: | Line 33: | ||
| | | | ||
| Staunton&Weissman2001 | | Staunton&Weissman2001 | ||
| − | |||
| | | | ||
| | | | ||
| Line 44: | Line 45: | ||
| Dewick2009 | | Dewick2009 | ||
| quinoline alkaloid, starter is Anthranilic acid | | quinoline alkaloid, starter is Anthranilic acid | ||
| − | |||
| | | | ||
| | | | ||
| Line 56: | Line 56: | ||
| Dewick2009 | | Dewick2009 | ||
| furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin and visnagin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin and visnagin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | ||
| − | |||
| Escherichia coli | | Escherichia coli | ||
| 10 | | 10 | ||
| Line 68: | Line 67: | ||
| Dewick2009 | | Dewick2009 | ||
| C-methylated analogue of orsellinic acid,the extra methyl is derived from SAM. | | C-methylated analogue of orsellinic acid,the extra methyl is derived from SAM. | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 79: | Line 77: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 91: | Line 88: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 104: | Line 100: | ||
| S. Gaisser, A. Trefzer, S. Stckert, A. Kirshning and A. Bechthold, J. Bacteriol., 1997, 179, 6271?6278.;Staunton&Weissman2001 | | S. Gaisser, A. Trefzer, S. Stckert, A. Kirshning and A. Bechthold, J. Bacteriol., 1997, 179, 6271?6278.;Staunton&Weissman2001 | ||
| lack 2 OH group | | lack 2 OH group | ||
| − | |||
| Saccharomyces cerevisiae, E. coli | | Saccharomyces cerevisiae, E. coli | ||
| 8 | | 8 | ||
| Line 115: | Line 110: | ||
| Streptomyces coelicolor | | Streptomyces coelicolor | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| Streptomyces parvulus | | Streptomyces parvulus | ||
| Line 127: | Line 121: | ||
| Hypericum perforatum | | Hypericum perforatum | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 140: | Line 133: | ||
| Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | ||
| Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin.The Aspergillus parasiticus polyketide synthase genepksA, a homolog of Aspergillus nidulans wA, is required for aflatoxin B1 biosynthesis | | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin.The Aspergillus parasiticus polyketide synthase genepksA, a homolog of Aspergillus nidulans wA, is required for aflatoxin B1 biosynthesis | ||
| − | |||
| | | | ||
| 14 | | 14 | ||
| Line 152: | Line 144: | ||
| Dewick2009 | | Dewick2009 | ||
| Hexanoate is the starter unit | | Hexanoate is the starter unit | ||
| − | |||
| | | | ||
| | | | ||
| Line 164: | Line 155: | ||
| Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | ||
| Norsolorinic acid and averufin are its precursors. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Norsolorinic acid and averufin are its precursors. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 11 | | 11 | ||
| Line 176: | Line 166: | ||
| Dewick2009 | | Dewick2009 | ||
| Hexanoate is the starter unit | | Hexanoate is the starter unit | ||
| − | |||
| | | | ||
| | | | ||
| Line 188: | Line 177: | ||
| Dewick2009 | | Dewick2009 | ||
| Hexanoate is the starter unit | | Hexanoate is the starter unit | ||
| − | |||
| | | | ||
| | | | ||
| Line 199: | Line 187: | ||
| Aloe ferox | | Aloe ferox | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 211: | Line 198: | ||
| | | | ||
| Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 224: | Line 210: | ||
| 2005,Fujii et al.An iterative type I polyketide synthase PKSN catalyzes synthesis of the decaketide alternapyrone with regio-specific octa-methylation.Chem Biol. | | 2005,Fujii et al.An iterative type I polyketide synthase PKSN catalyzes synthesis of the decaketide alternapyrone with regio-specific octa-methylation.Chem Biol. | ||
| decaketide-derived a-pyrone with eight methyl branches | | decaketide-derived a-pyrone with eight methyl branches | ||
| − | |||
| a-amylase promoter/A. oryzae | | a-amylase promoter/A. oryzae | ||
| 20 | | 20 | ||
| Line 236: | Line 221: | ||
| Herbert1989 | | Herbert1989 | ||
| biosynthesized from two polyketide chains. Contribute to disease development in the plant host by the fungus. Condensation if a hexaketide-derived acyl derivative with dihyfrotriacetic acid lactone. | | biosynthesized from two polyketide chains. Contribute to disease development in the plant host by the fungus. Condensation if a hexaketide-derived acyl derivative with dihyfrotriacetic acid lactone. | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 247: | Line 231: | ||
| Alternaria tenuis | | Alternaria tenuis | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 259: | Line 242: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 267: | Line 249: | ||
|- | |- | ||
| [[Mol:Andrimid .Mol|Andrimid ]] | | [[Mol:Andrimid .Mol|Andrimid ]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 284: | Line 265: | ||
| Herbert1989 | | Herbert1989 | ||
| structurally related to citrinin and and its biosynthesis similar. | | structurally related to citrinin and and its biosynthesis similar. | ||
| − | |||
| | | | ||
| | | | ||
| Line 296: | Line 276: | ||
| Dewick2009 | | Dewick2009 | ||
| FK520 | | FK520 | ||
| − | |||
| | | | ||
| | | | ||
| Line 308: | Line 287: | ||
| 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | | 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | ||
| dodecaketide | | dodecaketide | ||
| − | |||
| a-amylase promoter/A. oryzae | | a-amylase promoter/A. oryzae | ||
| | | | ||
| Line 320: | Line 298: | ||
| 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | | 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | ||
| undecaketide | | undecaketide | ||
| − | |||
| a-amylase promoter/A. oryzae | | a-amylase promoter/A. oryzae | ||
| | | | ||
| Line 331: | Line 308: | ||
| Aspergillus melleus | | Aspergillus melleus | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 343: | Line 319: | ||
| Aspergillus nidulans | | Aspergillus nidulans | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 356: | Line 331: | ||
| 2007,Bergmann et al.Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans.Nat Chem Biol.;Challis2008 | | 2007,Bergmann et al.Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans.Nat Chem Biol.;Challis2008 | ||
| PKS-NRPS hybrid metabolites | | PKS-NRPS hybrid metabolites | ||
| − | |||
| | | | ||
| | | | ||
| Line 367: | Line 341: | ||
| | | | ||
| Challis2008 | | Challis2008 | ||
| − | |||
| | | | ||
| | | | ||
| Line 379: | Line 352: | ||
| Aspergillus melleus | | Aspergillus melleus | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 392: | Line 364: | ||
| Herbert1989 ;Kruger,G.J.,Steyn,P.S.,Vleggaar,R.,andRabie,C.J.(1979).X-raycrystalstructureofasteltoxin,anovelmycotoxinfrom Aspergillusstellatus Curzi.J.Chem.Soc.Chem.Commun. 441?442. | | Herbert1989 ;Kruger,G.J.,Steyn,P.S.,Vleggaar,R.,andRabie,C.J.(1979).X-raycrystalstructureofasteltoxin,anovelmycotoxinfrom Aspergillusstellatus Curzi.J.Chem.Soc.Chem.Commun. 441?442. | ||
| linear a-pyrone-containingpolyketide. starter propionate and eight malonate; Or from acetate and methionine instead of propionate. Structurally related to citreoviridin and aurovertin | | linear a-pyrone-containingpolyketide. starter propionate and eight malonate; Or from acetate and methionine instead of propionate. Structurally related to citreoviridin and aurovertin | ||
| − | |||
| | | | ||
| | | | ||
| Line 403: | Line 374: | ||
| Aspergillus terreus | | Aspergillus terreus | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 415: | Line 385: | ||
| Penicillium sp, Aspergillus sp | | Penicillium sp, Aspergillus sp | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 427: | Line 396: | ||
| Penicillium sp, Aspergillus sp | | Penicillium sp, Aspergillus sp | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 440: | Line 408: | ||
| Herbert1989 | | Herbert1989 | ||
| mixed origins, from propionate and single acetate plus p-nitrobenzoic acid | | mixed origins, from propionate and single acetate plus p-nitrobenzoic acid | ||
| − | |||
| | | | ||
| | | | ||
| Line 450: | Line 417: | ||
| | | | ||
| Streptomyces thioluteus | | Streptomyces thioluteus | ||
| − | |||
| | | | ||
| | | | ||
| Line 464: | Line 430: | ||
| Herbert1989 ;1983J. Chem. Soc., Chem. Commun_Evidence for a mono-oxygenase mechanism in the biosynthesis of austdiol | | Herbert1989 ;1983J. Chem. Soc., Chem. Commun_Evidence for a mono-oxygenase mechanism in the biosynthesis of austdiol | ||
| Austdiol has a structure similar to citrinin and its biosynthesis appears to be similar. | | Austdiol has a structure similar to citrinin and its biosynthesis appears to be similar. | ||
| − | |||
| | | | ||
| | | | ||
| Line 476: | Line 441: | ||
| Herbert1989 | | Herbert1989 | ||
| from acetate/malonate plus succinyl CoA | | from acetate/malonate plus succinyl CoA | ||
| − | |||
| | | | ||
| | | | ||
| Line 488: | Line 452: | ||
| SanchezEtal2008;Dewick2009 | | SanchezEtal2008;Dewick2009 | ||
| Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 20 | | 20 | ||
| Line 500: | Line 463: | ||
| Dewick2009 | | Dewick2009 | ||
| macrolides | | macrolides | ||
| − | |||
| | | | ||
| | | | ||
| Line 512: | Line 474: | ||
| Dewick2009 | | Dewick2009 | ||
| macrolides | | macrolides | ||
| − | |||
| | | | ||
| | | | ||
| Line 524: | Line 485: | ||
| SanchezEtal2008;Dewick2009 | | SanchezEtal2008;Dewick2009 | ||
| Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 20 | | 20 | ||
| Line 535: | Line 495: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 548: | Line 507: | ||
| Herbert1989 | | Herbert1989 | ||
| one methyl group derives from methionine and the other by reduction of a carboxy-group. The ethyl group derives form C-2 of acetate and methionine. | | one methyl group derives from methionine and the other by reduction of a carboxy-group. The ethyl group derives form C-2 of acetate and methionine. | ||
| − | |||
| | | | ||
| 9 | | 9 | ||
| Line 560: | Line 518: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| probable intermediate of griseofulvin | | probable intermediate of griseofulvin | ||
| − | |||
| | | | ||
| 14 | | 14 | ||
| Line 572: | Line 529: | ||
| Herbert1989 | | Herbert1989 | ||
| nonaketide, from singal chain | | nonaketide, from singal chain | ||
| − | |||
| E.coli. | | E.coli. | ||
| 18 | | 18 | ||
| Line 584: | Line 540: | ||
| Herbert1989 | | Herbert1989 | ||
| like penicillic acid ,via orsellinic acid but cleavage occurs between C-3 and C-4 instead of C-4 and C-5, C-4 is lost at some stage | | like penicillic acid ,via orsellinic acid but cleavage occurs between C-3 and C-4 instead of C-4 and C-5, C-4 is lost at some stage | ||
| − | |||
| | | | ||
| | | | ||
| Line 596: | Line 551: | ||
| Herbert1989 | | Herbert1989 | ||
| a macrolide, different molecules of oxygen precludes a biosynthetic mechanism similar to prostaglandins. | | a macrolide, different molecules of oxygen precludes a biosynthetic mechanism similar to prostaglandins. | ||
| − | |||
| | | | ||
| 16 | | 16 | ||
| Line 608: | Line 562: | ||
| Dewick2009 | | Dewick2009 | ||
| polyether | | polyether | ||
| − | |||
| | | | ||
| | | | ||
| Line 620: | Line 573: | ||
| Dewick2009 | | Dewick2009 | ||
| polyether | | polyether | ||
| − | |||
| | | | ||
| | | | ||
| Line 627: | Line 579: | ||
|- | |- | ||
| [[Mol:C-1027.Mol|C-1027]] | | [[Mol:C-1027.Mol|C-1027]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 643: | Line 594: | ||
| Micromonospora echinospora | | Micromonospora echinospora | ||
| 2009,Belecki et al.Production of octaketide polyenes by the calicheamicin polyketide synthase CalE8: implications for the biosynthesis of enediyne core structures.J Am Chem Soc | | 2009,Belecki et al.Production of octaketide polyenes by the calicheamicin polyketide synthase CalE8: implications for the biosynthesis of enediyne core structures.J Am Chem Soc | ||
| − | |||
| | | | ||
| | | | ||
| Line 656: | Line 606: | ||
| Dewick2009 | | Dewick2009 | ||
| cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | ||
| − | |||
| | | | ||
| | | | ||
| Line 668: | Line 617: | ||
| Dewick2009 | | Dewick2009 | ||
| cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | ||
| − | |||
| | | | ||
| | | | ||
| Line 680: | Line 628: | ||
| Dewick2009 | | Dewick2009 | ||
| cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | ||
| − | |||
| | | | ||
| | | | ||
| Line 691: | Line 638: | ||
| Cercospora sp, Cercospora kikuchii | | Cercospora sp, Cercospora kikuchii | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 704: | Line 650: | ||
| Dewick2009 | | Dewick2009 | ||
| starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | ||
| − | |||
| | | | ||
| 19 | | 19 | ||
| Line 715: | Line 660: | ||
| Streptomyces sp | | Streptomyces sp | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 727: | Line 671: | ||
| Streptomyces sp | | Streptomyces sp | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 739: | Line 682: | ||
| Asahina chrysantha, Cassia angustifolia | | Asahina chrysantha, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 751: | Line 693: | ||
| Asahina chrysantha, Cassia angustifolia | | Asahina chrysantha, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 764: | Line 705: | ||
| Dewick2009 | | Dewick2009 | ||
| polyether | | polyether | ||
| − | |||
| | | | ||
| | | | ||
| Line 776: | Line 716: | ||
| 1981,Sylvie Rebuffat, Daniel Davoust and Darius Molho, Phytochemistry, Biosynthesis of citreomontanin in Penicillium pedemontanum | | 1981,Sylvie Rebuffat, Daniel Davoust and Darius Molho, Phytochemistry, Biosynthesis of citreomontanin in Penicillium pedemontanum | ||
| linear a-pyrone-containingpolyketide | | linear a-pyrone-containingpolyketide | ||
| − | |||
| | | | ||
| | | | ||
| Line 788: | Line 727: | ||
| Sakabe,N.,Goto,T.,andHirata,Y.(1977).Structureofcitreoviridin,amycotoxinproducedby Penicilliumcitreo-viride molded onrice.Tetrahedron 33,3077?3081.;Niwa,M.,Endo,T.,Ogiso,S.,Furukawa,H.,andYamamura,S. (1981).Twonewpyrones,metabolitesof Penicilliumcitreo-viride Biouge.Chem.Lett.(Jpn)1285?1288 | | Sakabe,N.,Goto,T.,andHirata,Y.(1977).Structureofcitreoviridin,amycotoxinproducedby Penicilliumcitreo-viride molded onrice.Tetrahedron 33,3077?3081.;Niwa,M.,Endo,T.,Ogiso,S.,Furukawa,H.,andYamamura,S. (1981).Twonewpyrones,metabolitesof Penicilliumcitreo-viride Biouge.Chem.Lett.(Jpn)1285?1288 | ||
| linear a-pyrone-containingpolyketide | | linear a-pyrone-containingpolyketide | ||
| − | |||
| | | | ||
| | | | ||
| Line 800: | Line 738: | ||
| Herbert1989 | | Herbert1989 | ||
| via a dihydro-isocoumarins | | via a dihydro-isocoumarins | ||
| − | |||
| | | | ||
| 10 | | 10 | ||
| Line 811: | Line 748: | ||
| Aspergillus clavatus | | Aspergillus clavatus | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 824: | Line 760: | ||
| Herbert1989 | | Herbert1989 | ||
| two carbons from methionine | | two carbons from methionine | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 836: | Line 771: | ||
| Herbert1989 ;1993Biosynthesis of colletodiol and related polyketide macrodiolides in Cytospora sp. ATCC 20502 : synthesis and metabolism of advanced intermediates | | Herbert1989 ;1993Biosynthesis of colletodiol and related polyketide macrodiolides in Cytospora sp. ATCC 20502 : synthesis and metabolism of advanced intermediates | ||
| non-aromatic,by tetraketide and triketide | | non-aromatic,by tetraketide and triketide | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 843: | Line 777: | ||
|- | |- | ||
| [[Mol:Compactin.Mol|Compactin]] | | [[Mol:Compactin.Mol|Compactin]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 860: | Line 793: | ||
| Herbert1989 | | Herbert1989 | ||
| similar in chemical structure to zearalenone | | similar in chemical structure to zearalenone | ||
| − | |||
| | | | ||
| 16 | | 16 | ||
| Line 872: | Line 804: | ||
| Herbert1989 | | Herbert1989 | ||
| fungal phenalenones | | fungal phenalenones | ||
| − | |||
| | | | ||
| 15 | | 15 | ||
| Line 883: | Line 814: | ||
| | | | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 895: | Line 825: | ||
| Aspergillus terreus | | Aspergillus terreus | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 908: | Line 837: | ||
| Herbert1989 ;1983, Charles P. Gorst-Allman, Pieter S. Steyn and Robert Vleggaar, Biosynthesis of diplosporin by Diplodia macrospora. Part 2. Investigation of ring formation using stable isotopes. J. Chem. Soc., Perkin Trans. 1,?1357 - 1359 | | Herbert1989 ;1983, Charles P. Gorst-Allman, Pieter S. Steyn and Robert Vleggaar, Biosynthesis of diplosporin by Diplodia macrospora. Part 2. Investigation of ring formation using stable isotopes. J. Chem. Soc., Perkin Trans. 1,?1357 - 1359 | ||
| two C1 units from methionine, C11 plus C12 constitute the starter acetate | | two C1 units from methionine, C11 plus C12 constitute the starter acetate | ||
| − | |||
| | | | ||
| 10 | | 10 | ||
| Line 919: | Line 847: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 932: | Line 859: | ||
| Dewick2009 | | Dewick2009 | ||
| macrolides | | macrolides | ||
| − | |||
| | | | ||
| | | | ||
| Line 939: | Line 865: | ||
|- | |- | ||
| [[Mol:Dothistromin.Mol|Dothistromin]] | | [[Mol:Dothistromin.Mol|Dothistromin]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 956: | Line 881: | ||
| Dewick2009 | | Dewick2009 | ||
| Adriamycin. anthracycline antibiotics,The starter group for the type II PKS is propionyl-CoA | | Adriamycin. anthracycline antibiotics,The starter group for the type II PKS is propionyl-CoA | ||
| − | |||
| | | | ||
| | | | ||
| Line 967: | Line 891: | ||
| Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 979: | Line 902: | ||
| Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 991: | Line 913: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,003: | Line 924: | ||
| Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,015: | Line 935: | ||
| Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,023: | Line 942: | ||
|- | |- | ||
| [[Mol:Enniatin B.Mol|Enniatin B]] | | [[Mol:Enniatin B.Mol|Enniatin B]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,038: | Line 956: | ||
| | | | ||
| Streptomyces maritimus | | Streptomyces maritimus | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,051: | Line 968: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,063: | Line 979: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,075: | Line 990: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,087: | Line 1,001: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,099: | Line 1,012: | ||
| Phyllosticta sp | | Phyllosticta sp | ||
| Herbert1989 ;1975 Biosynthesis of Epoxydon and Related Compounds by Phyllosticta sp. Agric. Bioi. Chem. ( Japan), 39,409-13 | | Herbert1989 ;1975 Biosynthesis of Epoxydon and Related Compounds by Phyllosticta sp. Agric. Bioi. Chem. ( Japan), 39,409-13 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,112: | Line 1,024: | ||
| Herbert1989 | | Herbert1989 | ||
| 9 acetate units. propionate starter unit is extended by malonate unit. | | 9 acetate units. propionate starter unit is extended by malonate unit. | ||
| − | |||
| | | | ||
| 20 | | 20 | ||
| Line 1,123: | Line 1,034: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,135: | Line 1,045: | ||
| Aspergillus flaviceps | | Aspergillus flaviceps | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,148: | Line 1,057: | ||
| Herbert1989 | | Herbert1989 | ||
| carboxy-group is lost after introduction of the C-5 hydroxy-group;otherwise a symmetrical intermediate would have been generated. | | carboxy-group is lost after introduction of the C-5 hydroxy-group;otherwise a symmetrical intermediate would have been generated. | ||
| − | |||
| | | | ||
| 7 | | 7 | ||
| Line 1,160: | Line 1,068: | ||
| | | | ||
| Nonaketide | | Nonaketide | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,172: | Line 1,079: | ||
| Herbert1989 | | Herbert1989 | ||
| propionate and two acetate | | propionate and two acetate | ||
| − | |||
| | | | ||
| 7 | | 7 | ||
| Line 1,179: | Line 1,085: | ||
|- | |- | ||
| [[Mol:Geldanamycin.Mol|Geldanamycin]] | | [[Mol:Geldanamycin.Mol|Geldanamycin]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,196: | Line 1,101: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| acetate-derived metabolite,Phenolic oxidative coupling | | acetate-derived metabolite,Phenolic oxidative coupling | ||
| − | |||
| | | | ||
| 14 | | 14 | ||
| Line 1,207: | Line 1,111: | ||
| Penicillium griseofulvin | | Penicillium griseofulvin | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,219: | Line 1,122: | ||
| Penicillium griseofulvin | | Penicillium griseofulvin | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,232: | Line 1,134: | ||
| Dewick2009 | | Dewick2009 | ||
| polyethers, macrolides | | polyethers, macrolides | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,244: | Line 1,145: | ||
| Dewick2009 | | Dewick2009 | ||
| typical bitter taste of beer, foam-stabilizing and antibacterial properties. Formed by oxidative transformation of deoxyhumulone. the starter unit for the polyketide is leucine-derived isovaleryl-CoA. | | typical bitter taste of beer, foam-stabilizing and antibacterial properties. Formed by oxidative transformation of deoxyhumulone. the starter unit for the polyketide is leucine-derived isovaleryl-CoA. | ||
| − | |||
| | | | ||
| 13 | | 13 | ||
| Line 1,256: | Line 1,156: | ||
| Dewick2009 | | Dewick2009 | ||
| Antidepressive agent in St John’s Wort Polyketide nature is almost entirely obscured by the added isoprenoid fragments | | Antidepressive agent in St John’s Wort Polyketide nature is almost entirely obscured by the added isoprenoid fragments | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 1,268: | Line 1,167: | ||
| Dewick2009 | | Dewick2009 | ||
| a constituent of St John’s Wort, Hypericum perforatum (Guttiferae/Hypericaceae). | | a constituent of St John’s Wort, Hypericum perforatum (Guttiferae/Hypericaceae). | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,280: | Line 1,178: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| anthraquinone. Islandicin is another anthraquinone pigment produced by Penicillium islandicum, and differs from emodin in two ways: one hydroxyl is missing and a new hydroxyl has been in corporated adjacent to the methyl. | | anthraquinone. Islandicin is another anthraquinone pigment produced by Penicillium islandicum, and differs from emodin in two ways: one hydroxyl is missing and a new hydroxyl has been in corporated adjacent to the methyl. | ||
| − | |||
| | | | ||
| 15 | | 15 | ||
| Line 1,290: | Line 1,187: | ||
| | | | ||
| Penicillium patulum (=Penicillium urticae) | | Penicillium patulum (=Penicillium urticae) | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,304: | Line 1,200: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism Different from usnic acid. using the alternative hydroxyl nucleophile in heterocyclic ring formation | | Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism Different from usnic acid. using the alternative hydroxyl nucleophile in heterocyclic ring formation | ||
| − | |||
| | | | ||
| 14 | | 14 | ||
| Line 1,316: | Line 1,211: | ||
| Dewick2009 | | Dewick2009 | ||
| macrolides | | macrolides | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,328: | Line 1,222: | ||
| Dewick2009 | | Dewick2009 | ||
| furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.PCS,Pentaketide chromone synthase, plant-speci?c type III PKS | | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.PCS,Pentaketide chromone synthase, plant-speci?c type III PKS | ||
| − | |||
| Escherichia coli | | Escherichia coli | ||
| 11 | | 11 | ||
| Line 1,340: | Line 1,233: | ||
| Dewick2009 | | Dewick2009 | ||
| polyether antibiotics | | polyether antibiotics | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,352: | Line 1,244: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| A depside (an ester formed from two phenolic acids). Combination of two orsellinic acid thioester molecule | | A depside (an ester formed from two phenolic acids). Combination of two orsellinic acid thioester molecule | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 1,363: | Line 1,254: | ||
| | | | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,376: | Line 1,266: | ||
| Herbert1989 | | Herbert1989 | ||
| chromanone, from two polyketide chain | | chromanone, from two polyketide chain | ||
| − | |||
| | | | ||
| 11 | | 11 | ||
| Line 1,388: | Line 1,277: | ||
| | | | ||
| Nonaketide.(mevinolin; monacolin K) | | Nonaketide.(mevinolin; monacolin K) | ||
| − | |||
| | | | ||
| yeast | | yeast | ||
| Line 1,400: | Line 1,288: | ||
| Dewick2009 | | Dewick2009 | ||
| methylphenol;3-Hydroxytoluene;3-cresol; derivative of 6-MSA | | methylphenol;3-Hydroxytoluene;3-cresol; derivative of 6-MSA | ||
| − | |||
| | | | ||
| 7 | | 7 | ||
| Line 1,412: | Line 1,299: | ||
| | | | ||
| Heptaketide | | Heptaketide | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,423: | Line 1,309: | ||
| Aspergillus melleus, Aspergillus ochraceus | | Aspergillus melleus, Aspergillus ochraceus | ||
| Herbert1989 ;2003_Natural Products_ the Secondary Metabolites (Tutorial Chemistry Texts) | | Herbert1989 ;2003_Natural Products_ the Secondary Metabolites (Tutorial Chemistry Texts) | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,436: | Line 1,321: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| Two molecules of methylphloracetophenone: usnic acid | | Two molecules of methylphloracetophenone: usnic acid | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 1,448: | Line 1,332: | ||
| Herbert1989 | | Herbert1989 | ||
| naphthoquinone.Two potential routes for the biosynthesis of mollisin: one chain and two chains | | naphthoquinone.Two potential routes for the biosynthesis of mollisin: one chain and two chains | ||
| − | |||
| | | | ||
| 11 | | 11 | ||
| Line 1,460: | Line 1,343: | ||
| Herbert1989 | | Herbert1989 | ||
| C31H52O9(R1)(R2),Monensin A (R1= -CH(CH3)COOH, R2= -CH2CH3) Monensin B (R1= -CH(CH3)COOH, R2= -CH3) Monensin C (R1= -(CH2)3COOH, R2= -CH2CH3) | | C31H52O9(R1)(R2),Monensin A (R1= -CH(CH3)COOH, R2= -CH2CH3) Monensin B (R1= -CH(CH3)COOH, R2= -CH3) Monensin C (R1= -(CH2)3COOH, R2= -CH2CH3) | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,472: | Line 1,354: | ||
| Herbert1989 ;1998,Application of Isotopic Methods to Secondary Metabolic Pathways. Thomas J.Simpson. Topics in Current Chemistry,Vol.195 | | Herbert1989 ;1998,Application of Isotopic Methods to Secondary Metabolic Pathways. Thomas J.Simpson. Topics in Current Chemistry,Vol.195 | ||
| fragmanted polyketide, The arrangement shown requires C-4,5 cleavae before loss of the carboxy-group. | | fragmanted polyketide, The arrangement shown requires C-4,5 cleavae before loss of the carboxy-group. | ||
| − | |||
| | | | ||
| 10 | | 10 | ||
| Line 1,482: | Line 1,363: | ||
| | | | ||
| Mycobacterium ulcerans | | Mycobacterium ulcerans | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,496: | Line 1,376: | ||
| Dewick2009;Herbert1989 | | Dewick2009;Herbert1989 | ||
| Immunosuppressive agent. 5-methylorsellinic acid is precursor. Addition of farnesyl alkyl. The chain length of the farnesyl alkyl group is subsequently shortened by oxidation of a double bond, giving demethylmycophenolic acid, which is then O-methylated, again involving SAM, to produce mycophenolic acid | | Immunosuppressive agent. 5-methylorsellinic acid is precursor. Addition of farnesyl alkyl. The chain length of the farnesyl alkyl group is subsequently shortened by oxidation of a double bond, giving demethylmycophenolic acid, which is then O-methylated, again involving SAM, to produce mycophenolic acid | ||
| − | |||
| | | | ||
| 12 | | 12 | ||
| Line 1,507: | Line 1,386: | ||
| Stigmatella aurantiaca | | Stigmatella aurantiaca | ||
| 2007,Pulsawat et al.Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae.Gene. | | 2007,Pulsawat et al.Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae.Gene. | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,518: | Line 1,396: | ||
| | | | ||
| Stigmatella aurantiaca | | Stigmatella aurantiaca | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,530: | Line 1,407: | ||
| | | | ||
| Stigmatella aurantiaca | | Stigmatella aurantiaca | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,542: | Line 1,418: | ||
| | | | ||
| Streptomyces caelestis | | Streptomyces caelestis | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,556: | Line 1,431: | ||
| SanchezEtal2008;Dewick2009 | | SanchezEtal2008;Dewick2009 | ||
| Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 20 | | 20 | ||
| Line 1,568: | Line 1,442: | ||
| Dewick2009 | | Dewick2009 | ||
| macrolide,(NYS; Mycostatin (TN)) | | macrolide,(NYS; Mycostatin (TN)) | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,580: | Line 1,453: | ||
| 1979,Huff and Hamilton, W.E. Huff and P.B. Hamilton, Mycotoxins?their biosynthesis in fungi: ochratoxins?metabolites of combined pathways, Journal of Food Protection 42 (1979), pp. 815?820.;2001Harris and Mantle, J.P. Harris and P.G. Mantle, Biosynthesis of ochratoxins by Aspergillus ochraceus, Phytochemistry 58 (2001), pp. 709?716;2009,Gallo et al.Characterisation of a pks gene which is expressed during ochratoxin A production by Aspergillus carbonarius.Int J Food Microbiol. | | 1979,Huff and Hamilton, W.E. Huff and P.B. Hamilton, Mycotoxins?their biosynthesis in fungi: ochratoxins?metabolites of combined pathways, Journal of Food Protection 42 (1979), pp. 815?820.;2001Harris and Mantle, J.P. Harris and P.G. Mantle, Biosynthesis of ochratoxins by Aspergillus ochraceus, Phytochemistry 58 (2001), pp. 709?716;2009,Gallo et al.Characterisation of a pks gene which is expressed during ochratoxin A production by Aspergillus carbonarius.Int J Food Microbiol. | ||
| OTA is a potent nephrotoxin and a possible human carcinogen with a polyketide derived structure.Structurally OTA consists of a polyketide which is believed to be derived from a dihydroiso-coumarin group that is amide-linked to the amino acid L-phenylalanine.Its biosynthesis pathway has yet not been completely elucidated, although a number of putative pathways have been proposed (Harris and Mantle, 2001; Huff and Hamilton, 1979). | | OTA is a potent nephrotoxin and a possible human carcinogen with a polyketide derived structure.Structurally OTA consists of a polyketide which is believed to be derived from a dihydroiso-coumarin group that is amide-linked to the amino acid L-phenylalanine.Its biosynthesis pathway has yet not been completely elucidated, although a number of putative pathways have been proposed (Harris and Mantle, 2001; Huff and Hamilton, 1979). | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,592: | Line 1,464: | ||
| Herbert1989 | | Herbert1989 | ||
| from two chains. formed by the condensation of two acetate-derived chains and the introduction of a C, unit (presumably from methionine) at C(4). | | from two chains. formed by the condensation of two acetate-derived chains and the introduction of a C, unit (presumably from methionine) at C(4). | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 1,604: | Line 1,475: | ||
| Dewick2009 | | Dewick2009 | ||
| polyether,PP1, PP2A inhibitor | | polyether,PP1, PP2A inhibitor | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,616: | Line 1,486: | ||
| Dewick2009 | | Dewick2009 | ||
| Amimycin; Landomycin; Matromycin; Romicil | | Amimycin; Landomycin; Matromycin; Romicil | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,628: | Line 1,497: | ||
| Dewick2009 | | Dewick2009 | ||
| A saturated C6 hexanoate starter unit | | A saturated C6 hexanoate starter unit | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,640: | Line 1,508: | ||
| Herbert1989 ; 1982, Application of?2H?-isotopic shifts in?13C n.m.r. spectra to biosynthetic studies. Incorporation of [1-13C,?2H3]acetate into?O-methylasparvenone in?Aspergillus parvulus. J. Chem. SOC., Chem. Commun.,1074. | | Herbert1989 ; 1982, Application of?2H?-isotopic shifts in?13C n.m.r. spectra to biosynthetic studies. Incorporation of [1-13C,?2H3]acetate into?O-methylasparvenone in?Aspergillus parvulus. J. Chem. SOC., Chem. Commun.,1074. | ||
| formed by the way of naphthalene | | formed by the way of naphthalene | ||
| − | |||
| | | | ||
| 12 | | 12 | ||
| Line 1,652: | Line 1,519: | ||
| Herbert1989 | | Herbert1989 | ||
| Collie’s realisation that the triketone might be anintermediate in orcinol biosynthesis was inspirational. synthesized chemically from dehydroacetic acid, likely via a polyketone intermediate,suggested that polyphenols could be biosynthesized from a C2 precursor | | Collie’s realisation that the triketone might be anintermediate in orcinol biosynthesis was inspirational. synthesized chemically from dehydroacetic acid, likely via a polyketone intermediate,suggested that polyphenols could be biosynthesized from a C2 precursor | ||
| − | |||
| | | | ||
| 7 | | 7 | ||
| Line 1,664: | Line 1,530: | ||
| Dewick2009;G.-L. Tang and W. Liu, Biochem. Biophys. Res. Commun., 2006,345, 133?139. | | Dewick2009;G.-L. Tang and W. Liu, Biochem. Biophys. Res. Commun., 2006,345, 133?139. | ||
| lack1OH group, because formation of conjugated system enolization | | lack1OH group, because formation of conjugated system enolization | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 1,676: | Line 1,541: | ||
| Dewick2009 | | Dewick2009 | ||
| starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | ||
| − | |||
| | | | ||
| 19 | | 19 | ||
| Line 1,688: | Line 1,552: | ||
| Herbert1989 | | Herbert1989 | ||
| nonaromatic six membered ring. C-12 was unexpectedly not labelled by 18O, but this is the consequence of ready exchange at this position | | nonaromatic six membered ring. C-12 was unexpectedly not labelled by 18O, but this is the consequence of ready exchange at this position | ||
| − | |||
| | | | ||
| 14 | | 14 | ||
| Line 1,700: | Line 1,563: | ||
| Dewick2009;1971,Aberhart and Caspi.The fate of the 6 alpha-hydrogen of 5 alpha-cholest-7-en-3 beta-ol in the conversion to 7-dehydrocholesterol by rat liver microsomes.J Biol Chem. | | Dewick2009;1971,Aberhart and Caspi.The fate of the 6 alpha-hydrogen of 5 alpha-cholest-7-en-3 beta-ol in the conversion to 7-dehydrocholesterol by rat liver microsomes.J Biol Chem. | ||
| derived from acetate via 6-methylsalicylic acid | | derived from acetate via 6-methylsalicylic acid | ||
| − | |||
| | | | ||
| 6 | | 6 | ||
| Line 1,712: | Line 1,574: | ||
| Herbert1989; Dewick2009 | | Herbert1989; Dewick2009 | ||
| This time orsellinic acid is aprecursor, and ring fission appears to proceed viaa quinone, which is the result of decarboxylation,oxidation, and methylation reactions | | This time orsellinic acid is aprecursor, and ring fission appears to proceed viaa quinone, which is the result of decarboxylation,oxidation, and methylation reactions | ||
| − | |||
| | | | ||
| 6 | | 6 | ||
| Line 1,722: | Line 1,583: | ||
| | | | ||
| Aspergillus clavatus | | Aspergillus clavatus | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,736: | Line 1,596: | ||
| Dewick2009 | | Dewick2009 | ||
| analogue of phloroacetophenone. But using isobutyryl-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA. type III PKS. using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | | analogue of phloroacetophenone. But using isobutyryl-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA. type III PKS. using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,748: | Line 1,607: | ||
| Dewick2009 | | Dewick2009 | ||
| using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | | using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,759: | Line 1,617: | ||
| Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | ||
| Dewick2009; | | Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,771: | Line 1,628: | ||
| Streptomyces venezuelae | | Streptomyces venezuelae | ||
| Herbert1989 | | Herbert1989 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,779: | Line 1,635: | ||
|- | |- | ||
| [[Mol:Pimaricin.Mol|Pimaricin]] | | [[Mol:Pimaricin.Mol|Pimaricin]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,796: | Line 1,651: | ||
| Herbert1989 | | Herbert1989 | ||
| The platenomycins are closely related to leucomycin | | The platenomycins are closely related to leucomycin | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,808: | Line 1,662: | ||
| Dewick2009 | | Dewick2009 | ||
| Malonamyl-CoA is the starter unit.Tetracyclic backbone with all carbon atoms from malonated derived precursor of tetracycline | | Malonamyl-CoA is the starter unit.Tetracyclic backbone with all carbon atoms from malonated derived precursor of tetracycline | ||
| − | |||
| | | | ||
| 19 | | 19 | ||
| Line 1,819: | Line 1,672: | ||
| Streptomyces natalensis | | Streptomyces natalensis | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,830: | Line 1,682: | ||
| | | | ||
| Pseudomonas aeruginosa | | Pseudomonas aeruginosa | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,839: | Line 1,690: | ||
|- | |- | ||
| [[Mol:Pyoluteorin.Mol|Pyoluteorin]] | | [[Mol:Pyoluteorin.Mol|Pyoluteorin]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,856: | Line 1,706: | ||
| Herbert1989 | | Herbert1989 | ||
| formed from two polyketide chains; alternatively they may be formed by a pathway involving ring-cleavage. | | formed from two polyketide chains; alternatively they may be formed by a pathway involving ring-cleavage. | ||
| − | |||
| | | | ||
| 11 | | 11 | ||
| Line 1,867: | Line 1,716: | ||
| Streptomyces hygroscopicus | | Streptomyces hygroscopicus | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,880: | Line 1,728: | ||
| Herbert1989 | | Herbert1989 | ||
| xanthone,Islandicin is plausibly an intermediate in ravenilin biosynthesis | | xanthone,Islandicin is plausibly an intermediate in ravenilin biosynthesis | ||
| − | |||
| | | | ||
| 12 | | 12 | ||
| Line 1,891: | Line 1,738: | ||
| Cassia angustifolia | | Cassia angustifolia | ||
| 2002,Dewick.Medicinal natural products : a biosynthetic approach.Wiley;Dewick2009; | | 2002,Dewick.Medicinal natural products : a biosynthetic approach.Wiley;Dewick2009; | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,903: | Line 1,749: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,915: | Line 1,760: | ||
| Amycolatopsis mediterranei | | Amycolatopsis mediterranei | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,927: | Line 1,771: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,940: | Line 1,783: | ||
| Herbert1989 | | Herbert1989 | ||
| fungal alpha-pyrones. Two carbons are derived from methionine | | fungal alpha-pyrones. Two carbons are derived from methionine | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 1,947: | Line 1,789: | ||
|- | |- | ||
| [[Mol:Rubrofusarin.Mol|Rubrofusarin]] | | [[Mol:Rubrofusarin.Mol|Rubrofusarin]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 1,964: | Line 1,805: | ||
| Herbert1989 | | Herbert1989 | ||
| Rubropunctatin can be split into two fragments on the basis of labelling results. The left-hand part derives in the usual way. The left-habd part -though seems to derive through the beta-keto acid formed by condensation of hexanoic acid with an acetate unit. | | Rubropunctatin can be split into two fragments on the basis of labelling results. The left-hand part derives in the usual way. The left-habd part -though seems to derive through the beta-keto acid formed by condensation of hexanoic acid with an acetate unit. | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 1,976: | Line 1,816: | ||
| Herbert1989 | | Herbert1989 | ||
| unusual, from two polyketide chains | | unusual, from two polyketide chains | ||
| − | |||
| | | | ||
| 9 | | 9 | ||
| Line 1,988: | Line 1,827: | ||
| Herbert1989 | | Herbert1989 | ||
| from one polyketide chains | | from one polyketide chains | ||
| − | |||
| | | | ||
| 10 | | 10 | ||
| Line 2,000: | Line 1,838: | ||
| Dewick2009 | | Dewick2009 | ||
| A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | | A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | ||
| − | |||
| | | | ||
| 16 | | 16 | ||
| Line 2,012: | Line 1,849: | ||
| Dewick2009 | | Dewick2009 | ||
| A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | | A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | ||
| − | |||
| | | | ||
| 16 | | 16 | ||
| Line 2,024: | Line 1,860: | ||
| Dewick2009 | | Dewick2009 | ||
| macrolides | | macrolides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,036: | Line 1,871: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,048: | Line 1,882: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,060: | Line 1,893: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,072: | Line 1,904: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,084: | Line 1,915: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,096: | Line 1,926: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,108: | Line 1,937: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,120: | Line 1,948: | ||
| Dewick2009 | | Dewick2009 | ||
| dianthrone O-glycosides | | dianthrone O-glycosides | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,132: | Line 1,959: | ||
| | | | ||
| fungal tropolone | | fungal tropolone | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,144: | Line 1,970: | ||
| Herbert1989 | | Herbert1989 | ||
| Antifungal drug,The fungal metabolite siccanin (21) contains a sesquiterpenoid fragment and a fragment derived from orsellinic acid. | | Antifungal drug,The fungal metabolite siccanin (21) contains a sesquiterpenoid fragment and a fragment derived from orsellinic acid. | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,156: | Line 1,981: | ||
| Herbert1989 ;1985 CPB_Biosynthesis of Silvaticamide, a Toxin from Aspergillus silvaticus | | Herbert1989 ;1985 CPB_Biosynthesis of Silvaticamide, a Toxin from Aspergillus silvaticus | ||
| derived by fragmentation of an anthraquinone, have similar prenylated skeleton with tajixanthone | | derived by fragmentation of an anthraquinone, have similar prenylated skeleton with tajixanthone | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 2,168: | Line 1,992: | ||
| Herbert1989 | | Herbert1989 | ||
| nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | ||
| − | |||
| E.coli (pET24/BL21(DE3)) | | E.coli (pET24/BL21(DE3)) | ||
| 18 | | 18 | ||
| Line 2,180: | Line 2,003: | ||
| Herbert1989 | | Herbert1989 | ||
| nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | ||
| − | |||
| E.coli (pET24/BL21(DE3)) | | E.coli (pET24/BL21(DE3)) | ||
| | | | ||
| Line 2,192: | Line 2,014: | ||
| Herbert1989 | | Herbert1989 | ||
| nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | ||
| − | |||
| E.coli (pET24/BL21(DE3)) | | E.coli (pET24/BL21(DE3)) | ||
| | | | ||
| Line 2,202: | Line 2,023: | ||
| | | | ||
| Sorangium cellulosum | | Sorangium cellulosum | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,214: | Line 2,034: | ||
| | | | ||
| Aspergillus fumigatus | | Aspergillus fumigatus | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,223: | Line 2,042: | ||
|- | |- | ||
| [[Mol:Spiramycin I.Mol|Spiramycin I]] | | [[Mol:Spiramycin I.Mol|Spiramycin I]] | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,240: | Line 2,058: | ||
| Yabe&Nakajima2004;Dewick2009;2009,Hertweck.The biosynthetic logic of polyketide diversity.Angew Chem Int Ed Engl. | | Yabe&Nakajima2004;Dewick2009;2009,Hertweck.The biosynthetic logic of polyketide diversity.Angew Chem Int Ed Engl. | ||
| Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 15 | | 15 | ||
| Line 2,250: | Line 2,067: | ||
| | | | ||
| Stigmatella aurantiaca | | Stigmatella aurantiaca | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,264: | Line 2,080: | ||
| Herbert1989 ;1963,Bentley.Biosynthesis of tropolones in Penicillium stipitatum.” J Biol Chem. | | Herbert1989 ;1963,Bentley.Biosynthesis of tropolones in Penicillium stipitatum.” J Biol Chem. | ||
| 3-methylorsellinic acid is a intermediate, tropolone containing metabolite | | 3-methylorsellinic acid is a intermediate, tropolone containing metabolite | ||
| − | |||
| | | | ||
| 9 | | 9 | ||
| Line 2,276: | Line 2,091: | ||
| Herbert1989 | | Herbert1989 | ||
| via the anthraquinone. fungal p-methylbenzophenone derivatives | | via the anthraquinone. fungal p-methylbenzophenone derivatives | ||
| − | |||
| | | | ||
| 13 | | 13 | ||
| Line 2,287: | Line 2,101: | ||
| Streptomyces sp | | Streptomyces sp | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,300: | Line 2,113: | ||
| Herbert1989 | | Herbert1989 | ||
| derived by fragmentation of an anthraquinone, have similar prenylated skeleton with silvaticamide | | derived by fragmentation of an anthraquinone, have similar prenylated skeleton with silvaticamide | ||
| − | |||
| | | | ||
| 20 | | 20 | ||
| Line 2,312: | Line 2,124: | ||
| | | | ||
| fungal PKS-NRPS | | fungal PKS-NRPS | ||
| − | |||
| a-amylase promoter/A. oryzae | | a-amylase promoter/A. oryzae | ||
| | | | ||
| Line 2,324: | Line 2,135: | ||
| Herbert1989 | | Herbert1989 | ||
| via a dihydro-isocoumarins; biosynthesis pathway is incompleted | | via a dihydro-isocoumarins; biosynthesis pathway is incompleted | ||
| − | |||
| | | | ||
| 8 | | 8 | ||
| Line 2,336: | Line 2,146: | ||
| 2007,Corre and Challis.Heavy tools for genome mining.Chem Biol.;[8]2007,Bouhired et al.Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA.Fungal Genet Biol. | | 2007,Corre and Challis.Heavy tools for genome mining.Chem Biol.;[8]2007,Bouhired et al.Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA.Fungal Genet Biol. | ||
| cryptic NRPS system | | cryptic NRPS system | ||
| − | |||
| TdiA-E in E. coli | | TdiA-E in E. coli | ||
| | | | ||
| Line 2,348: | Line 2,157: | ||
| 2008,Ames et al.Crystal structure and functional analysis of tetracenomycin ARO/CYC: implications for cyclization specificity of aromatic polyketides.Proc Natl Acad Sci U S A. | | 2008,Ames et al.Crystal structure and functional analysis of tetracenomycin ARO/CYC: implications for cyclization specificity of aromatic polyketides.Proc Natl Acad Sci U S A. | ||
| type II polyketide products | | type II polyketide products | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,360: | Line 2,168: | ||
| Dewick2009 | | Dewick2009 | ||
| starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | ||
| − | |||
| | | | ||
| 19 | | 19 | ||
| Line 2,372: | Line 2,179: | ||
| Dewick2009 | | Dewick2009 | ||
| Dronabinol; delta9-Tetrahydrocannabinol; Tetrahydrocannabinol.A saturated C6 hexanoate starter unit The aromatic ring/C5 chain originates from hexanoate and malonate, then cyclization.Precursor isolivetolic acid | | Dronabinol; delta9-Tetrahydrocannabinol; Tetrahydrocannabinol.A saturated C6 hexanoate starter unit The aromatic ring/C5 chain originates from hexanoate and malonate, then cyclization.Precursor isolivetolic acid | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,384: | Line 2,190: | ||
| 2001,Pfeifer and Khosla.Biosynthesis of polyketides in heterologous hosts.Microbiol Mol Biol Rev.;2007,American Chemical Society. Meeting (229th : 2005 : San Diego Calif.) et al.Polyketides : biosynthesis, biological activity, and genetic engineering.American Chemical Society : Distributed by Oxford University Press | | 2001,Pfeifer and Khosla.Biosynthesis of polyketides in heterologous hosts.Microbiol Mol Biol Rev.;2007,American Chemical Society. Meeting (229th : 2005 : San Diego Calif.) et al.Polyketides : biosynthesis, biological activity, and genetic engineering.American Chemical Society : Distributed by Oxford University Press | ||
| intermediate of napyradiomycin A | | intermediate of napyradiomycin A | ||
| − | |||
| Aspergillus oryzae | | Aspergillus oryzae | ||
| 10 | | 10 | ||
| Line 2,396: | Line 2,201: | ||
| 2005BB_Microbial Synthesis of Triacetic Acid Lactone | | 2005BB_Microbial Synthesis of Triacetic Acid Lactone | ||
| Ether ring formation by cyclization of the 3,5-diketohexanoate thioester ;not only one pks can produce TAL | | Ether ring formation by cyclization of the 3,5-diketohexanoate thioester ;not only one pks can produce TAL | ||
| − | |||
| Escherichia coli and Saccharomyces cerevisiae | | Escherichia coli and Saccharomyces cerevisiae | ||
| 6 | | 6 | ||
| Line 2,406: | Line 2,210: | ||
| | | | ||
| Aspergillus fumigatus | | Aspergillus fumigatus | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,418: | Line 2,221: | ||
| | | | ||
| Cochliobolus heterostrophus | | Cochliobolus heterostrophus | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,430: | Line 2,232: | ||
| | | | ||
| Streptomyces fradiae | | Streptomyces fradiae | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,443: | Line 2,244: | ||
| Streptomyces fradiae | | Streptomyces fradiae | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,456: | Line 2,256: | ||
| Dewick2009 | | Dewick2009 | ||
| 3-(8,11-Pentadecadienyl)-1,2-benzenediol. palmitoleoyl-CoA ( 9-hexadecenoyl-CoA) can act as starter group for extension by three malonyl-CoA units | | 3-(8,11-Pentadecadienyl)-1,2-benzenediol. palmitoleoyl-CoA ( 9-hexadecenoyl-CoA) can act as starter group for extension by three malonyl-CoA units | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,468: | Line 2,267: | ||
| Dewick2009; | | Dewick2009; | ||
| antibacterial metabolite.formed by two methylphloracetophenone. Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism. c-methylation by SAM | | antibacterial metabolite.formed by two methylphloracetophenone. Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism. c-methylation by SAM | ||
| − | |||
| | | | ||
| 14(8,8) | | 14(8,8) | ||
| Line 2,480: | Line 2,278: | ||
| Herbert1989 | | Herbert1989 | ||
| from acetate(malonate) and methionine with the pyrrolidone ring having its origins in glutamic acid | | from acetate(malonate) and methionine with the pyrrolidone ring having its origins in glutamic acid | ||
| − | |||
| | | | ||
| 12 | | 12 | ||
| Line 2,492: | Line 2,289: | ||
| Yabe&Nakajima2004;Dewick2009 | | Yabe&Nakajima2004;Dewick2009 | ||
| Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 2,504: | Line 2,300: | ||
| Yabe&Nakajima2004;Dewick2009 | | Yabe&Nakajima2004;Dewick2009 | ||
| Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 2,516: | Line 2,311: | ||
| Yabe&Nakajima2004;Dewick2009 | | Yabe&Nakajima2004;Dewick2009 | ||
| Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 2,528: | Line 2,322: | ||
| Yabe&Nakajima2004;Dewick2009 | | Yabe&Nakajima2004;Dewick2009 | ||
| Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | ||
| − | |||
| | | | ||
| 17 | | 17 | ||
| Line 2,540: | Line 2,333: | ||
| Dewick2009 | | Dewick2009 | ||
| furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | ||
| − | |||
| Escherichia coli | | Escherichia coli | ||
| 11 | | 11 | ||
| Line 2,550: | Line 2,342: | ||
| wA | | wA | ||
| Aspergillus nidulans | | Aspergillus nidulans | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,562: | Line 2,353: | ||
| | | | ||
| Sporomiella intermedia, Leptodontium elatius | | Sporomiella intermedia, Leptodontium elatius | ||
| − | |||
| | | | ||
| | | | ||
| Line 2,575: | Line 2,365: | ||
| | | | ||
| Dewick2009 | | Dewick2009 | ||
| − | |||
| | | | ||
| | | | ||
Revision as of 01:35, 7 March 2010
| name | PKS | gene | organism | Ref | Note | Size | C2 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1,3,8-Trihydroxyaceto-Naphthalene | Aspergillus parvulus | Herbert1989 | Compound name have not been confirmed. Naphthalene is the base structure of statins. | 12 | 6 | 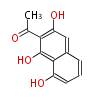
| |||
| 1,3-Dihydroxy-N-Methylacridone | Acridone synthase | Dewick2009 | acridine alkaloid, starter is Anthranilic acid | 3 | 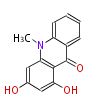
| ||||
| 3,8-Dihydroxy-1-Methylanthraquinone-2-Carboxylic Acid | Staunton&Weissman2001 | 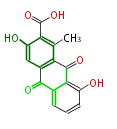
| |||||||
| 4-Hydroxy-2-Quinolone | Dewick2009 | quinoline alkaloid, starter is Anthranilic acid | 1 | 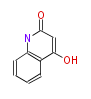
| |||||
| 5,7-Dihydroxy-2-Methylchromone | Pentaketide chromone synthase | Ammi visnaga | Dewick2009 | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin and visnagin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | Escherichia coli | 10 | 5 | 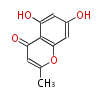
| |
| 5-Methylorsellinic Acid | Aspergillus flaviceps | Dewick2009 | C-methylated analogue of orsellinic acid,the extra methyl is derived from SAM. | 8 | 4 | 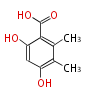
| |||
| 6-Deoxy-Erythronolide B | DEBS | Dewick2009 | 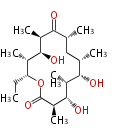
| ||||||
| 6-Methoxymellein | Dewick2009 | 5 | 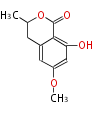
| ||||||
| 6-Methyl Salicylic Acid | 6-MSAS/P. patulum(ATX/Aspergillus terreus) | atX | Penicillium patulum, Aspergillus terreus | S. Gaisser, A. Trefzer, S. Stckert, A. Kirshning and A. Bechthold, J. Bacteriol., 1997, 179, 6271?6278.;Staunton&Weissman2001 | lack 2 OH group | Saccharomyces cerevisiae, E. coli | 8 | 4 | 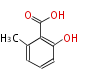
|
| Actinorhodin | act | Streptomyces coelicolor | Herbert1989 | Streptomyces parvulus | 16 | 8 | 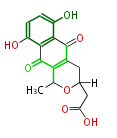
| ||
| Adhyperforin | Hypericum perforatum | Dewick2009 | 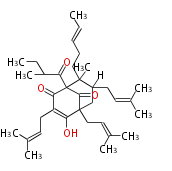
| ||||||
| Aflatoxin B1 | PKSL1/Aspergillus parasiticus=PKSA | pksL1/Aspergillus parasiticus (Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB,AflN,AflM,OmtB,OmtA,OrdA) | Aspergillus flavus, Aspergillus parasiticus | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin.The Aspergillus parasiticus polyketide synthase genepksA, a homolog of Aspergillus nidulans wA, is required for aflatoxin B1 biosynthesis | 14 | 10 | 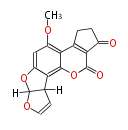
| |
| Aflatoxin B2 | Aspergillus flavus, Aspergillus parasiticus | Dewick2009 | Hexanoate is the starter unit | 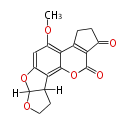
| |||||
| Aflatoxin G1 | (Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB,AflN,AflM,OmtB,OmtA,OrdA) | Aspergillus flavus, Aspergillus parasiticus | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 11 | 10 | 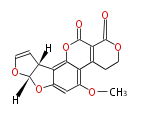
| ||
| Aflatoxin G2 | Aspergillus flavus, Aspergillus parasiticus | Dewick2009 | Hexanoate is the starter unit | 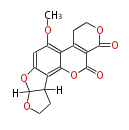
| |||||
| Aflatoxin M1 | Aspergillus flavus, Aspergillus parasiticus | Dewick2009 | Hexanoate is the starter unit | 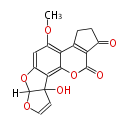
| |||||
| Aloe-Emodin | Aloe ferox | Dewick2009; | 15 | 8 | 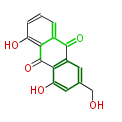
| ||||
| Aloesaponarin II | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | 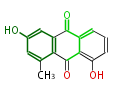
| |||||||
| Alternapyrone | PKSN | alt5 from Alternaria solani | 2005,Fujii et al.An iterative type I polyketide synthase PKSN catalyzes synthesis of the decaketide alternapyrone with regio-specific octa-methylation.Chem Biol. | decaketide-derived a-pyrone with eight methyl branches | a-amylase promoter/A. oryzae | 20 | 10 | 
| |
| Alternaric Acid | Alternaria solani, Alternaria alternata (formerly known as A. kikuchiana) | Herbert1989 | biosynthesized from two polyketide chains. Contribute to disease development in the plant host by the fungus. Condensation if a hexaketide-derived acyl derivative with dihyfrotriacetic acid lactone. | 17 | 6 | 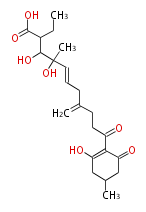
| |||
| Alternariol | Alternaria tenuis | Herbert1989 | 14 | 7 | 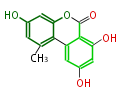
| ||||
| Amphotericin B | Dewick2009 | 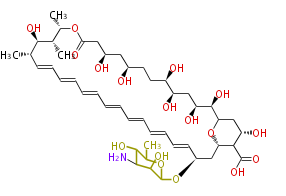
| |||||||
| Andrimid | 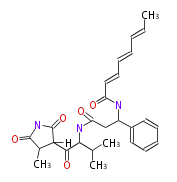
| ||||||||
| Aschochitin | Herbert1989 | structurally related to citrinin and and its biosynthesis similar. | File:Aschochitin.Mol.png | ||||||
| Ascomycin | Dewick2009 | FK520 | 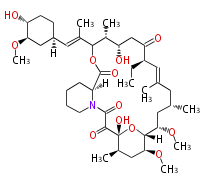
| ||||||
| Aslaniol | PKSF from A. solani | 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | dodecaketide | a-amylase promoter/A. oryzae | 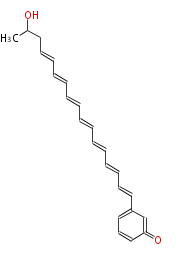
| ||||
| Aslanipyrone | PKSF from A. solani | 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | undecaketide | a-amylase promoter/A. oryzae | 
| ||||
| Asperlactone | Aspergillus melleus | Herbert1989 | 8 | 5 | 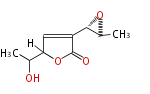
| ||||
| Asperlin | Aspergillus nidulans | Herbert1989 | 8 | 5 | 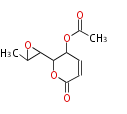
| ||||
| Aspyridone A | Aspergillus nidulans | 2007,Bergmann et al.Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans.Nat Chem Biol.;Challis2008 | PKS-NRPS hybrid metabolites | 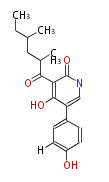
| |||||
| Aspyridone B | Challis2008 | 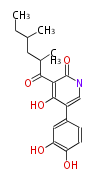
| |||||||
| Aspyrone | Aspergillus melleus | Herbert1989 | 7 | 5 | 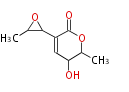
| ||||
| Asteltoxin | Aspergillus stellatus | Herbert1989 ;Kruger,G.J.,Steyn,P.S.,Vleggaar,R.,andRabie,C.J.(1979).X-raycrystalstructureofasteltoxin,anovelmycotoxinfrom Aspergillusstellatus Curzi.J.Chem.Soc.Chem.Commun. 441?442. | linear a-pyrone-containingpolyketide. starter propionate and eight malonate; Or from acetate and methionine instead of propionate. Structurally related to citreoviridin and aurovertin | 9 | 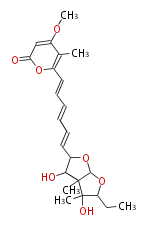
| ||||
| Astepyrone | Aspergillus terreus | Herbert1989 | 4 | 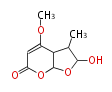
| |||||
| Atrochrysone | Penicillium sp, Aspergillus sp | Dewick2009; | 15 | 8 | 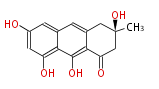
| ||||
| Atrochrysone Aarboxylic Acid | Penicillium sp, Aspergillus sp | Dewick2009; | 16 | 8 | File:Atrochrysone Aarboxylic Acid.Mol.png | ||||
| Aureothin | Streptomyces thioluteus | Herbert1989 | mixed origins, from propionate and single acetate plus p-nitrobenzoic acid | 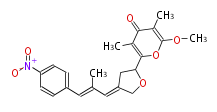
| |||||
| Aurovertin | Streptomyces thioluteus | File:Aurovertin.Mol.png | |||||||
| Austdiol | Herbert1989 ;1983J. Chem. Soc., Chem. Commun_Evidence for a mono-oxygenase mechanism in the biosynthesis of austdiol | Austdiol has a structure similar to citrinin and its biosynthesis appears to be similar. | 5 | 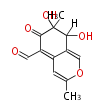
| |||||
| Avenaciolide | Herbert1989 | from acetate/malonate plus succinyl CoA | 
| ||||||
| Averantin | Nor-1;StcE | Aspergillus parasiticus, Aspergillus flavus | SanchezEtal2008;Dewick2009 | Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 20 | 10 | 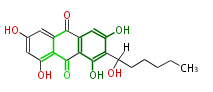
| ||
| Avermectin B1a | Dewick2009 | macrolides | 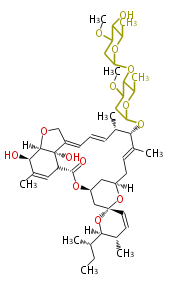
| ||||||
| Avermectin B2a | Dewick2009 | macrolides | 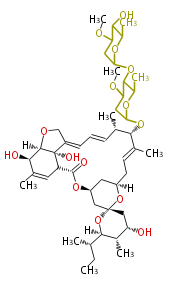
| ||||||
| Averufin | Fas1,Fas2,PksA,Nor-1,AvnA,AdhA | Aspergillus parasiticus, Aspergillus flavus | SanchezEtal2008;Dewick2009 | Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 20 | 10 | 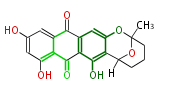
| ||
| Avilamycin | Dewick2009 | 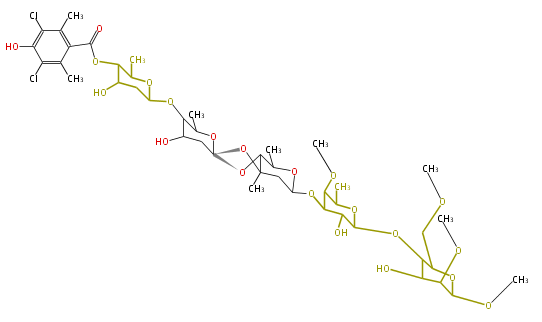
| |||||||
| Barnol | Penicillium baarnense | Herbert1989 | one methyl group derives from methionine and the other by reduction of a carboxy-group. The ethyl group derives form C-2 of acetate and methionine. | 9 | 4 | 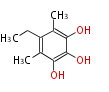
| |||
| Benzophenone | Penicillium griseofulvin | Dewick2009;Herbert1989 | probable intermediate of griseofulvin | 14 | 7 | 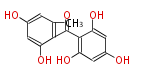
| |||
| Bikaverin | Bikaverin nonaketide synthase | PKS4 from Gibberella fujikuroi | Gibberella fujikuroi | Herbert1989 | nonaketide, from singal chain | E.coli. | 18 | 9 | 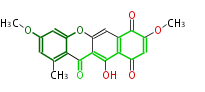
|
| Botryodiplodin | Herbert1989 | like penicillic acid ,via orsellinic acid but cleavage occurs between C-3 and C-4 instead of C-4 and C-5, C-4 is lost at some stage | 4 | 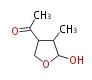
| |||||
| Brefeldin A | Penicillium decumbens, Penicillium brefeldianum, Penicillium cyaneum, Aspergillus clavatus,Eupenicillium brefeldianum, | Herbert1989 | a macrolide, different molecules of oxygen precludes a biosynthetic mechanism similar to prostaglandins. | 16 | 8 | 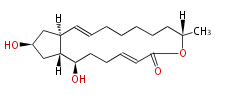
| |||
| Brevetoxin A | Karenia brevis | Dewick2009 | polyether | 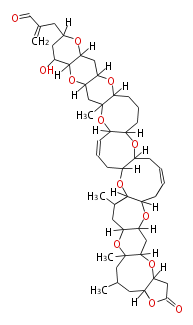
| |||||
| Brevetoxin B | Karenia brevis | Dewick2009 | polyether | 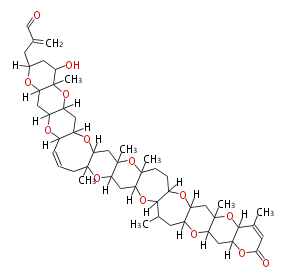
| |||||
| C-1027 | 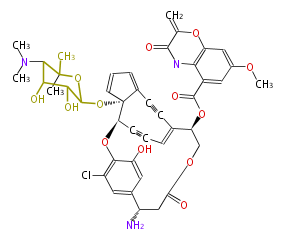
| ||||||||
| Calichemicin Gamma1 | Micromonospora echinospora | 2009,Belecki et al.Production of octaketide polyenes by the calicheamicin polyketide synthase CalE8: implications for the biosynthesis of enediyne core structures.J Am Chem Soc | 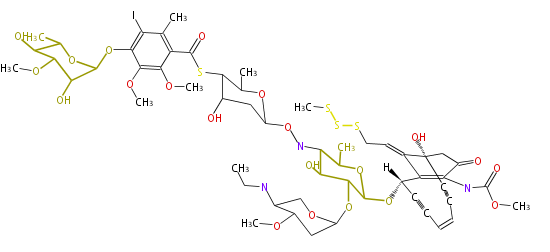
| ||||||
| Cannabidiol | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | 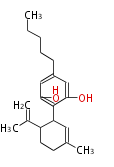
| |||||
| Cannabigerolic Acid | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | 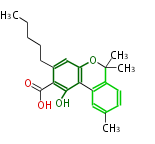
| |||||
| Cannabinol | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | 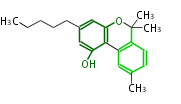
| |||||
| Cercosporin | Cercospora sp, Cercospora kikuchii | Herbert1989 | 7 | 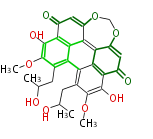
| |||||
| Chlortetracycline | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI,OxyF,OxyL,OxyG,OxyQ,OxyR,OxyT,OxyS,OxyE,TchA | Streptomyces rimosus, Streptomyces aureofaciens | Dewick2009 | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | 19 | 8 | 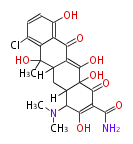
| |
| Chrysomycin A | Streptomyces sp | Herbert1989 | 17 | 9 | 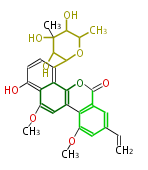
| ||||
| Chrysomycin B | Streptomyces sp | Herbert1989 | 17 | 9 | 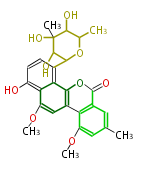
| ||||
| Chrysophanol | Asahina chrysantha, Cassia angustifolia | Dewick2009; | 15 | 8 | 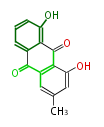
| ||||
| Chrysophanol Anthrone | Asahina chrysantha, Cassia angustifolia | Dewick2009; | 15 | 8 | 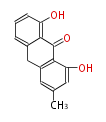
| ||||
| Ciguatoxin I | Gymnothorax javanicus, Lutjanus bohar | Dewick2009 | polyether | 
| |||||
| Citreomontanin | Penicillium pedemontanum | 1981,Sylvie Rebuffat, Daniel Davoust and Darius Molho, Phytochemistry, Biosynthesis of citreomontanin in Penicillium pedemontanum | linear a-pyrone-containingpolyketide | 
| |||||
| Citreoviridin | Sakabe,N.,Goto,T.,andHirata,Y.(1977).Structureofcitreoviridin,amycotoxinproducedby Penicilliumcitreo-viride molded onrice.Tetrahedron 33,3077?3081.;Niwa,M.,Endo,T.,Ogiso,S.,Furukawa,H.,andYamamura,S. (1981).Twonewpyrones,metabolitesof Penicilliumcitreo-viride Biouge.Chem.Lett.(Jpn)1285?1288 | linear a-pyrone-containingpolyketide | 
| ||||||
| Citrinin | Penicillium citrinum | Herbert1989 | via a dihydro-isocoumarins | 10 | 5 | 
| |||
| Clavatol | Aspergillus clavatus | Herbert1989 | 8 | 4 | 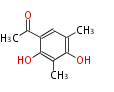
| ||||
| Coarctatin | Herbert1989 | two carbons from methionine | 8 | 4 | 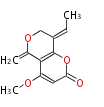
| ||||
| Colletodiol | Cytospora spp | Herbert1989 ;1993Biosynthesis of colletodiol and related polyketide macrodiolides in Cytospora sp. ATCC 20502 : synthesis and metabolism of advanced intermediates | non-aromatic,by tetraketide and triketide | 8 | 7(4+3) | 
| |||
| Compactin | 
| ||||||||
| Curvularin | Penicillium sp FP1768, Penicillium baradicum Hellllinrll Osporiulll | Herbert1989 | similar in chemical structure to zearalenone | 16 | 8 | 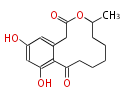
| |||
| Deoxyherquienone | Herbert1989 | fungal phenalenones | 15 | 7 | 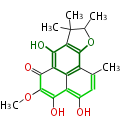
| ||||
| Deoxyradicin | Herbert1989 | 11 | 6 | 
| |||||
| Dihydroisocoumarin | Aspergillus terreus | Herbert1989 | 10 | 5 | 
| ||||
| Diplosporin | Diplodia macrospora | Herbert1989 ;1983, Charles P. Gorst-Allman, Pieter S. Steyn and Robert Vleggaar, Biosynthesis of diplosporin by Diplodia macrospora. Part 2. Investigation of ring formation using stable isotopes. J. Chem. Soc., Perkin Trans. 1,?1357 - 1359 | two C1 units from methionine, C11 plus C12 constitute the starter acetate | 10 | 5 | 
| |||
| Discodermolide | Dewick2009 | 
| |||||||
| Doramectin | Dewick2009 | macrolides | 
| ||||||
| Dothistromin | 
| ||||||||
| Doxorubicin | Streptomyces peuceticus | Dewick2009 | Adriamycin. anthracycline antibiotics,The starter group for the type II PKS is propionyl-CoA | 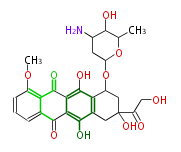
| |||||
| Emodin | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 15 | 8 | 
| ||||
| Emodin Anthrone | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 15 | 8 | 
| ||||
| Emodin Dianthrone | Dewick2009 | 
| |||||||
| Endocrocin | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 16 | 8 | 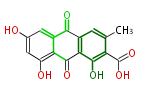
| ||||
| Endocrocin Anthrone | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 16 | 8 | 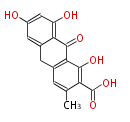
| ||||
| Enniatin B | 
| ||||||||
| Enterocin | Streptomyces maritimus | 
| |||||||
| Epothilone A | Dewick2009 | 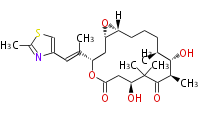
| |||||||
| Epothilone B | Dewick2009 | 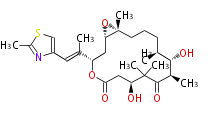
| |||||||
| Epothilone C | Dewick2009 | 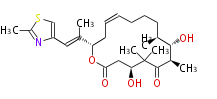
| |||||||
| Epothilone D | Dewick2009 | 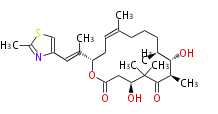
| |||||||
| Epoxydon | Phyllosticta sp | Herbert1989 ;1975 Biosynthesis of Epoxydon and Related Compounds by Phyllosticta sp. Agric. Bioi. Chem. ( Japan), 39,409-13 | 7 | 4 | 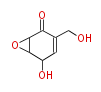
| ||||
| Epsilon-Pyrromycinone | Herbert1989 | 9 acetate units. propionate starter unit is extended by malonate unit. | 20 | 9 | 
| ||||
| Erythromycin | Dewick2009 | 
| |||||||
| Flavipin | Aspergillus flaviceps | Herbert1989 | 8 | 4 | 
| ||||
| Fumigatin | Aspergillus fumigatus | Herbert1989 | carboxy-group is lost after introduction of the C-5 hydroxy-group;otherwise a symmetrical intermediate would have been generated. | 7 | 4 | 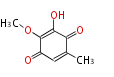
| |||
| Fumonisin B1 | FUM5 | Fusarium verticillioides, Gibberella fujikuroi | Nonaketide | 
| |||||
| Furanomycin | Herbert1989 | propionate and two acetate | 7 | 3 | 
| ||||
| Geldanamycin | 
| ||||||||
| Griseofulvin | Penicillium griseofulvin | Dewick2009;Herbert1989 | acetate-derived metabolite,Phenolic oxidative coupling | 14 | 7 | 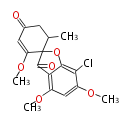
| |||
| Griseophenone B | Penicillium griseofulvin | Dewick2009;Herbert1989 | 14 | 7 | 
| ||||
| Griseophenone C | Penicillium griseofulvin | Dewick2009;Herbert1989 | 14 | 7 | 
| ||||
| Halichondrin B | Halichondria okadai | Dewick2009 | polyethers, macrolides | 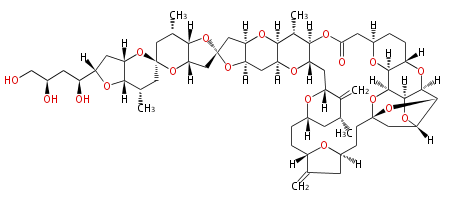
| |||||
| Humulone | Humulus lululus (hop; Cannabaceae) | Dewick2009 | typical bitter taste of beer, foam-stabilizing and antibacterial properties. Formed by oxidative transformation of deoxyhumulone. the starter unit for the polyketide is leucine-derived isovaleryl-CoA. | 13 | 3 | 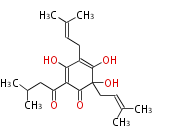
| |||
| Hyperforin | Hypericum perforatum | Dewick2009 | Antidepressive agent in St John’s Wort Polyketide nature is almost entirely obscured by the added isoprenoid fragments | 17 | 3 | 
| |||
| Hypericin | Dermocybe spp | Dewick2009 | a constituent of St John’s Wort, Hypericum perforatum (Guttiferae/Hypericaceae). | 
| |||||
| Islandicin | Penicillium islandicum | Dewick2009;Herbert1989 | anthraquinone. Islandicin is another anthraquinone pigment produced by Penicillium islandicum, and differs from emodin in two ways: one hydroxyl is missing and a new hydroxyl has been in corporated adjacent to the methyl. | 15 | 8 | 
| |||
| Isoepoxydon | Penicillium patulum (=Penicillium urticae) | 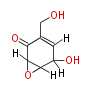
| |||||||
| Isousnic Acid | Usnea spp, Cladonia spp | Dewick2009;Herbert1989 | Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism Different from usnic acid. using the alternative hydroxyl nucleophile in heterocyclic ring formation | 14 | 4 | 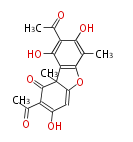
| |||
| Ivermectin | Dewick2009 | macrolides | 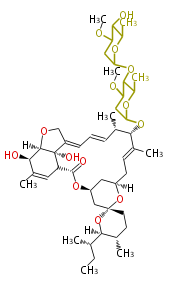
| ||||||
| Khellin | Pentaketide chromone synthase | Ammi visnaga | Dewick2009 | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.PCS,Pentaketide chromone synthase, plant-speci?c type III PKS | Escherichia coli | 11 | 5 | 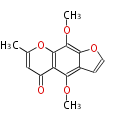
| |
| Lasalocid | Streptomyces lasaliensis | Dewick2009 | polyether antibiotics | 
| |||||
| Lecanoric Acid | Umbilicaria arctica, Umbilicaria nylanderiana | Dewick2009;Herbert1989 | A depside (an ester formed from two phenolic acids). Combination of two orsellinic acid thioester molecule | 8 | 4 | 
| |||
| Leucomycin A1 | Herbert1989 | 
| |||||||
| LL-D253alpha | Phoma pigmentivora | Herbert1989 | chromanone, from two polyketide chain | 11 | 6 | 
| |||
| Lovastatin | LOVB=LNKS, LovF=LDKS | loVF | Aspergillus terreus | Nonaketide.(mevinolin; monacolin K) | yeast | 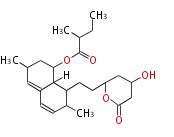
| |||
| M-Cresol | Aspergillus fumigatus | Dewick2009 | methylphenol;3-Hydroxytoluene;3-cresol; derivative of 6-MSA | 7 | 4 | 
| |||
| Melanin | ALB1=PKSP,PKS1 | Aspergillus fumigatus, Nodulisporium sp | Heptaketide | 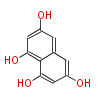
| |||||
| Mellein | Aspergillus melleus, Aspergillus ochraceus | Herbert1989 ;2003_Natural Products_ the Secondary Metabolites (Tutorial Chemistry Texts) | 10 | 5 | 
| ||||
| Methylphloracetophenone | Usnea spp, Cladonia spp | Dewick2009;Herbert1989 | Two molecules of methylphloracetophenone: usnic acid | 8 | 4 | 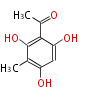
| |||
| Mollisin | Mollisia caesia | Herbert1989 | naphthoquinone.Two potential routes for the biosynthesis of mollisin: one chain and two chains | 11 | 8(8,3+5) | 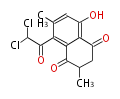
| |||
| Monensin A | Streptomyces cinnamonensis | Herbert1989 | C31H52O9(R1)(R2),Monensin A (R1= -CH(CH3)COOH, R2= -CH2CH3) Monensin B (R1= -CH(CH3)COOH, R2= -CH3) Monensin C (R1= -(CH2)3COOH, R2= -CH2CH3) | 
| |||||
| Multicolic Acid | Penicillium multicolor | Herbert1989 ;1998,Application of Isotopic Methods to Secondary Metabolic Pathways. Thomas J.Simpson. Topics in Current Chemistry,Vol.195 | fragmanted polyketide, The arrangement shown requires C-4,5 cleavae before loss of the carboxy-group. | 10 | 6 | 
| |||
| Mycolactone | Mycobacterium ulcerans | 
| |||||||
| Mycophenolic Acid | Penicillium brevicompactum | Dewick2009;Herbert1989 | Immunosuppressive agent. 5-methylorsellinic acid is precursor. Addition of farnesyl alkyl. The chain length of the farnesyl alkyl group is subsequently shortened by oxidation of a double bond, giving demethylmycophenolic acid, which is then O-methylated, again involving SAM, to produce mycophenolic acid | 12 | 4 | 
| |||
| Myxalamide | MxaC-1 | Stigmatella aurantiaca | 2007,Pulsawat et al.Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae.Gene. | 
| |||||
| Myxothiazol A | Stigmatella aurantiaca | 
| |||||||
| Myxothiazol Z | Stigmatella aurantiaca | 
| |||||||
| Niddamycin | Streptomyces caelestis | 
| |||||||
| Norsolorinic Acid | norsolorinic acid(NA) PKS | A?A/B(A.parasiticus); StcJ/K(A.nidulans) | Aspergillus parasiticus, Aspergillus flavus | SanchezEtal2008;Dewick2009 | Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 20 | 10 | 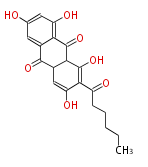
| |
| Nystatin | Streptomyces noursei | Dewick2009 | macrolide,(NYS; Mycostatin (TN)) | 
| |||||
| Ochratoxin A | otapks of A. ochraceus; otapksPN gene from P. nordicum; ACpks from A. carbonarius | Aspergillus ochraceus, Penicillium viridicatum,Penicillium verrucosum, Penicillium nordicum, Aspergillus fumigatus, Aspergillus carbonarius | 1979,Huff and Hamilton, W.E. Huff and P.B. Hamilton, Mycotoxins?their biosynthesis in fungi: ochratoxins?metabolites of combined pathways, Journal of Food Protection 42 (1979), pp. 815?820.;2001Harris and Mantle, J.P. Harris and P.G. Mantle, Biosynthesis of ochratoxins by Aspergillus ochraceus, Phytochemistry 58 (2001), pp. 709?716;2009,Gallo et al.Characterisation of a pks gene which is expressed during ochratoxin A production by Aspergillus carbonarius.Int J Food Microbiol. | OTA is a potent nephrotoxin and a possible human carcinogen with a polyketide derived structure.Structurally OTA consists of a polyketide which is believed to be derived from a dihydroiso-coumarin group that is amide-linked to the amino acid L-phenylalanine.Its biosynthesis pathway has yet not been completely elucidated, although a number of putative pathways have been proposed (Harris and Mantle, 2001; Huff and Hamilton, 1979). | 
| ||||
| Ochrephilone | Penicillium multicolor | Herbert1989 | from two chains. formed by the condensation of two acetate-derived chains and the introduction of a C, unit (presumably from methionine) at C(4). | 17 | 10(8+2) | 
| |||
| Okadaic Acid | Dinophysis sp, Prorocentrum lima | Dewick2009 | polyether,PP1, PP2A inhibitor | 
| |||||
| Oleandomycin | Streptomyces antibioticus | Dewick2009 | Amimycin; Landomycin; Matromycin; Romicil | 
| |||||
| Olivetolic Acid | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | A saturated C6 hexanoate starter unit | 4 | 
| ||||
| O-Methylasparvenone | Aspergillus parvulus | Herbert1989 ; 1982, Application of?2H?-isotopic shifts in?13C n.m.r. spectra to biosynthetic studies. Incorporation of [1-13C,?2H3]acetate into?O-methylasparvenone in?Aspergillus parvulus. J. Chem. SOC., Chem. Commun.,1074. | formed by the way of naphthalene | 12 | 6 | 
| |||
| Orcinol | Aspergillus fumigatus | Herbert1989 | Collie’s realisation that the triketone might be anintermediate in orcinol biosynthesis was inspirational. synthesized chemically from dehydroacetic acid, likely via a polyketone intermediate,suggested that polyphenols could be biosynthesized from a C2 precursor | 7 | 2 | 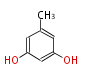
| |||
| Orsellinic Acid | Orsellinic acid synthase (OSAS) | aviM | Streptomyces viridochromogenes, Penicillium madriti | Dewick2009;G.-L. Tang and W. Liu, Biochem. Biophys. Res. Commun., 2006,345, 133?139. | lack1OH group, because formation of conjugated system enolization | 8 | 4 | 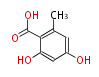
| |
| Oxytetracycline | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI,OxyF,OxyL,OxyG,OxyQ,OxyR,OxyT,OxyS,OxyE,TchA | Streptomyces rimosus, Streptomyces aureofaciens | Dewick2009 | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | 19 | 10 | 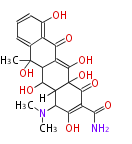
| |
| Palitantin | Penicillium palitans | Herbert1989 | nonaromatic six membered ring. C-12 was unexpectedly not labelled by 18O, but this is the consequence of ready exchange at this position | 14 | 7 | 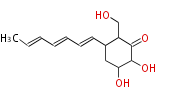
| |||
| Patulin | MSAS;ATX(=MSAS) | Aspergillus clavatus,Aspergillus terreus,Penicillium patulum (=Penicillium urticae) | Dewick2009;1971,Aberhart and Caspi.The fate of the 6 alpha-hydrogen of 5 alpha-cholest-7-en-3 beta-ol in the conversion to 7-dehydrocholesterol by rat liver microsomes.J Biol Chem. | derived from acetate via 6-methylsalicylic acid | 6 | 4 | 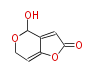
| ||
| Penicillic Acid | Penicillium cyclopium, Penicillium baarnense | Herbert1989; Dewick2009 | This time orsellinic acid is aprecursor, and ring fission appears to proceed viaa quinone, which is the result of decarboxylation,oxidation, and methylation reactions | 6 | 4 | 
| |||
| Phloracetophenone | Aspergillus clavatus | 8 | 4 | 
| |||||
| Phlorisobutyrophenone | Isobutyrophenonesynthase(BUS) | Hypericum calycinum | Dewick2009 | analogue of phloroacetophenone. But using isobutyryl-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA. type III PKS. using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | 3 | 
| |||
| Phlorisovalerophenone | phlorisovalerophenone synthase(VPS) | Humulus lululus (hop; Cannabaceae) | Dewick2009 | using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | 3 | 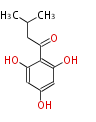
| |||
| Physcion | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 15 | 8 | 
| ||||
| Picromycin | Streptomyces venezuelae | Herbert1989 | 
| ||||||
| Pimaricin | 
| ||||||||
| Platenomycin | Herbert1989 | The platenomycins are closely related to leucomycin | File:Platenomycin.Mol.png | ||||||
| Pretetramide | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI | Streptomyces rimosus,Streptomyces aureofaciens | Dewick2009 | Malonamyl-CoA is the starter unit.Tetracyclic backbone with all carbon atoms from malonated derived precursor of tetracycline | 19 | 9 | 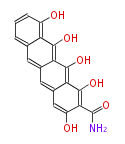
| |
| Protohypericin | Streptomyces natalensis | Dewick2009 | 
| ||||||
| Pyochelin | Pseudomonas aeruginosa | 
| |||||||
| Pyoluteorin | 
| ||||||||
| Radicin | Herbert1989 | formed from two polyketide chains; alternatively they may be formed by a pathway involving ring-cleavage. | 11 | 6 | 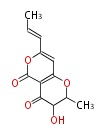
| ||||
| Rapamycin | Streptomyces hygroscopicus | Dewick2009 | 
| ||||||
| Ravenilin | Helminthosporium ravenelii, Helminthosporium turcicum | Herbert1989 | xanthone,Islandicin is plausibly an intermediate in ravenilin biosynthesis | 12 | 8 | 
| |||
| Rhein | Cassia angustifolia | 2002,Dewick.Medicinal natural products : a biosynthetic approach.Wiley;Dewick2009; | 15 | 8 | 
| ||||
| Rifabutin | Dewick2009 | 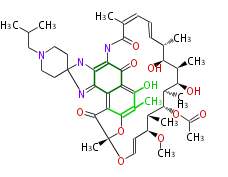
| |||||||
| Rifamycin B | Amycolatopsis mediterranei | Dewick2009 | 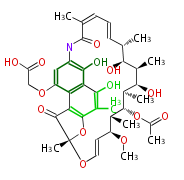
| ||||||
| Rifapentine | Dewick2009 | 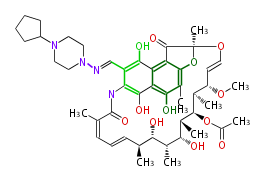
| |||||||
| Rosellisin | Herbert1989 | fungal alpha-pyrones. Two carbons are derived from methionine | 8 | 4 | 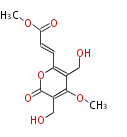
| ||||
| Rubrofusarin | 
| ||||||||
| Rubropunctatin | Monascus pilosus | Herbert1989 | Rubropunctatin can be split into two fragments on the basis of labelling results. The left-hand part derives in the usual way. The left-habd part -though seems to derive through the beta-keto acid formed by condensation of hexanoic acid with an acetate unit. | 17 | 6 | 
| |||
| Sclerin | Sclerotinia spp | Herbert1989 | unusual, from two polyketide chains | 9 | 5 | 
| |||
| Sclerotinin A | Sclerotinia spp | Herbert1989 | from one polyketide chains | 10 | 5 | 
| |||
| SEK4 | octaketide synthase OKS | Escherichia coli | Dewick2009 | A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | 16 | 8 | 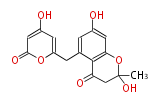
| ||
| SEK4b | octaketide synthase OKS | Escherichia coli | Dewick2009 | A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | 16 | 8 | 
| ||
| Selamectin | Dewick2009 | macrolides | 
| ||||||
| Sennidin A | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 
| ||||
| Sennidin B | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 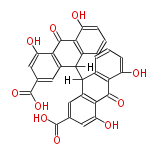
| ||||
| Sennidin C | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 
| ||||
| Sennidin D | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 
| ||||
| Sennosides A | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 
| ||||
| Sennosides B | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 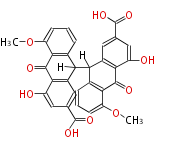
| ||||
| Sennosides C | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 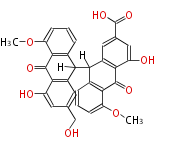
| ||||
| Sennosides D | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 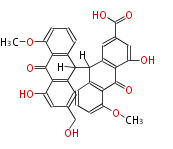
| ||||
| Sepedonin | fungal tropolone | 5 | 
| ||||||
| Siccanin | Helminthosporium siccans | Herbert1989 | Antifungal drug,The fungal metabolite siccanin (21) contains a sesquiterpenoid fragment and a fragment derived from orsellinic acid. | 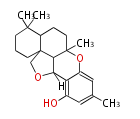
| |||||
| Silvaticamide | Aspergillus silvaticus | Herbert1989 ;1985 CPB_Biosynthesis of Silvaticamide, a Toxin from Aspergillus silvaticus | derived by fragmentation of an anthraquinone, have similar prenylated skeleton with tajixanthone | 17 | 8(12) | 
| |||
| SMA76a | pks4 from Gibberlla fujikuroi | Herbert1989 | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | E.coli (pET24/BL21(DE3)) | 18 | 9 | 
| ||
| SMA76b | pks4 from Gibberlla fujikuroi | Herbert1989 | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | E.coli (pET24/BL21(DE3)) | 9 | 
| |||
| SMA76c | pks4 from Gibberlla fujikuroi | Herbert1989 | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | E.coli (pET24/BL21(DE3)) | 9 | 
| |||
| Soraphen A | Sorangium cellulosum | 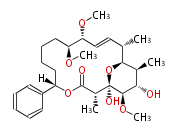
| |||||||
| Spinulosin | Aspergillus fumigatus | 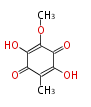
| |||||||
| Spiramycin I | 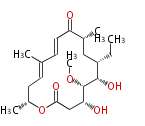
| ||||||||
| Sterigmatocystin | NSAS,PKS-st(STCA) | Aspergillus nidulans(pksST)(stcA)(Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB,AflN,AflM,OmtB) | Aspergillus parasiticus, Aspergillus flavus, Aspergillus nidulans | Yabe&Nakajima2004;Dewick2009;2009,Hertweck.The biosynthetic logic of polyketide diversity.Angew Chem Int Ed Engl. | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 15 | 10 | 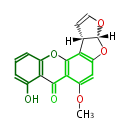
| |
| Stigmatellin A | Stigmatella aurantiaca | 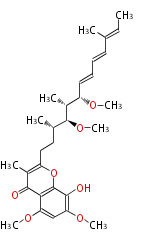
| |||||||
| Stipitatonic Acid | Penicillium stipitatum | Herbert1989 ;1963,Bentley.Biosynthesis of tropolones in Penicillium stipitatum.” J Biol Chem. | 3-methylorsellinic acid is a intermediate, tropolone containing metabolite | 9 | 4 | 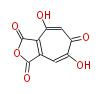
| |||
| Sulochrin | Aspergillus terreus, Aspergillus fumigatus | Herbert1989 | via the anthraquinone. fungal p-methylbenzophenone derivatives | 13 | 8 | 
| |||
| Tacrolimus | Streptomyces sp | Dewick2009 | 
| ||||||
| Tajixanthone | Aspergillus variecolor | Herbert1989 | derived by fragmentation of an anthraquinone, have similar prenylated skeleton with silvaticamide | 20 | 8(12) | 
| |||
| Tennellin | fungal PKS-NRPS | a-amylase promoter/A. oryzae | 
| ||||||
| Terrein | Aspergillus terreus, Aspergillus fumigatus | Herbert1989 | via a dihydro-isocoumarins; biosynthesis pathway is incompleted | 8 | 5 | 
| |||
| Terrequinone A | Aspergillus nidulans | 2007,Corre and Challis.Heavy tools for genome mining.Chem Biol.;[8]2007,Bouhired et al.Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA.Fungal Genet Biol. | cryptic NRPS system | TdiA-E in E. coli | 
| ||||
| Tetracenomycin F2 | tcm | Streptomyces glaucescens | 2008,Ames et al.Crystal structure and functional analysis of tetracenomycin ARO/CYC: implications for cyclization specificity of aromatic polyketides.Proc Natl Acad Sci U S A. | type II polyketide products | 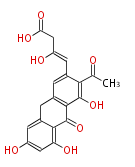
| ||||
| Tetracycline | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI,OxyF,OxyL,OxyG,OxyQ,OxyR,OxyT,OxyS,OxyE,TchA | Streptomyces rimosus,Streptomyces aureofaciens | Dewick2009 | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | 19 | 9 | 
| |
| Tetrahydro Cannabinol | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | Dronabinol; delta9-Tetrahydrocannabinol; Tetrahydrocannabinol.A saturated C6 hexanoate starter unit The aromatic ring/C5 chain originates from hexanoate and malonate, then cyclization.Precursor isolivetolic acid | 
| |||||
| Tetrahydroxynaphthalene | tetrahydroxynaphthalene synthase (THNS);RppA | pks1, from Colletotrichum lagenarium | Streptomyces griseus, Streptomyces coelicolor, Colletotrichum lagenarium | 2001,Pfeifer and Khosla.Biosynthesis of polyketides in heterologous hosts.Microbiol Mol Biol Rev.;2007,American Chemical Society. Meeting (229th : 2005 : San Diego Calif.) et al.Polyketides : biosynthesis, biological activity, and genetic engineering.American Chemical Society : Distributed by Oxford University Press | intermediate of napyradiomycin A | Aspergillus oryzae | 10 | 5 | 
|
| Triacetic Acid Lactone | 2-pyrone synthase | Gerbera hybrida | 2005BB_Microbial Synthesis of Triacetic Acid Lactone | Ether ring formation by cyclization of the 3,5-diketohexanoate thioester ;not only one pks can produce TAL | Escherichia coli and Saccharomyces cerevisiae | 6 | 3 | 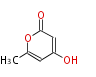
| |
| Trypacidin | Aspergillus fumigatus | 
| |||||||
| T-Toxin | PKS1 | Cochliobolus heterostrophus | 
| ||||||
| Tylactone | Streptomyces fradiae | 
| |||||||
| Tylosin | Streptomyces fradiae | Dewick2009 | 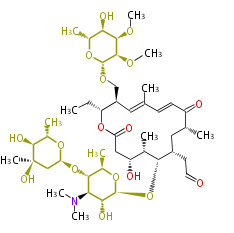
| ||||||
| Urushiol III | Toxicodendron radicans,Toxicodendron toxicaria | Dewick2009 | 3-(8,11-Pentadecadienyl)-1,2-benzenediol. palmitoleoyl-CoA ( 9-hexadecenoyl-CoA) can act as starter group for extension by three malonyl-CoA units | 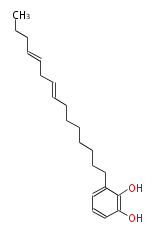
| |||||
| Usnic Acid | Usnea spp, Cladonia spp, Flavocetraria cucculata, Flavocetraria nivalis | Dewick2009; | antibacterial metabolite.formed by two methylphloracetophenone. Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism. c-methylation by SAM | 14(8,8) | 8(4,4) | 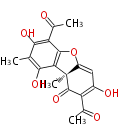
| |||
| Variotin | Herbert1989 | from acetate(malonate) and methionine with the pyrrolidone ring having its origins in glutamic acid | 12 | 6 | 
| ||||
| Versicolorin A | Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | 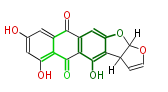
| ||
| Versicolorin B | Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | 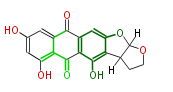
| ||
| Versiconal | Fas1,Fas2,PksA,Nor-1,AvnA,AdhA,AvfA,EstA | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | | ||
| Versiconal Acetate | Fas1,Fas2,PksA,Nor-1,AvnA,AdhA,AvfA | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | | ||
| Visnagin | Pentaketide chromone synthase | Ammi visnaga | Dewick2009 | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | Escherichia coli | 11 | 5 | 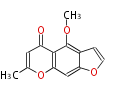
| |
| WA-Naphthopyron | wA | Aspergillus nidulans | 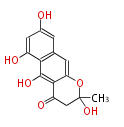
| ||||||
| Zaragozic Acids A | Sporomiella intermedia, Leptodontium elatius | 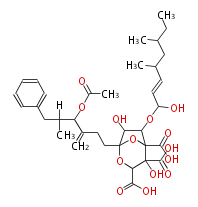
| |||||||
| Zearalenone | Dewick2009 | 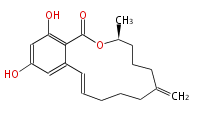
|